Validation process
Important
The Competent Authority (CA) user must have a confirmer role to be able to validate CI/PS applications and acknowledge PMCF/PMPF notifications.
Note
The validation process focuses on the administrative aspects of the application.
Once the sponsor submits or updates an application or notification (first submission or resubmission), the concerned CA receives a notification.
Once the sponsor submits an application or notification for additional country(ies), two notifications are sent:
To the Competent Authority(ies) in charge of validating the new application or in charge of acknowledging the new notification
To any Competent Authority involved in previous applications/notifications that have the same EU SIN
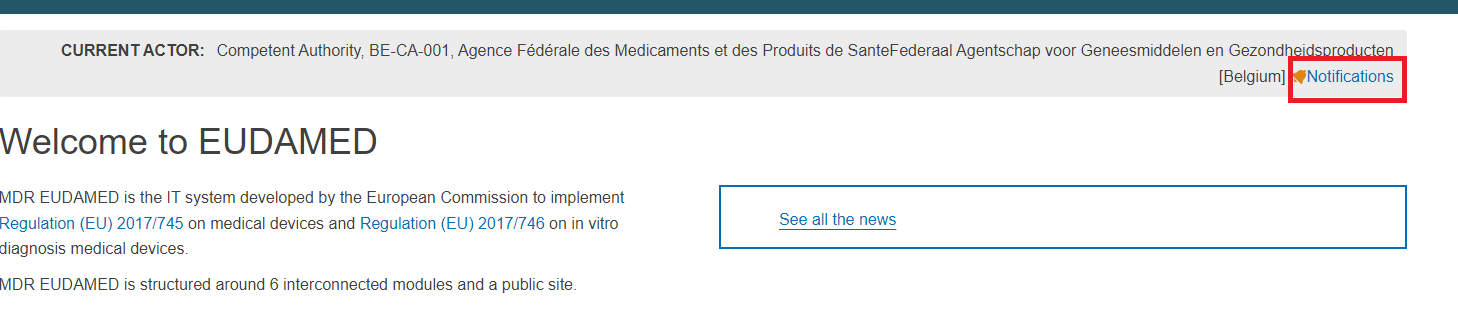
To check all notifications:
At the top of the Welcome to EUDAMED page click on Notifications:
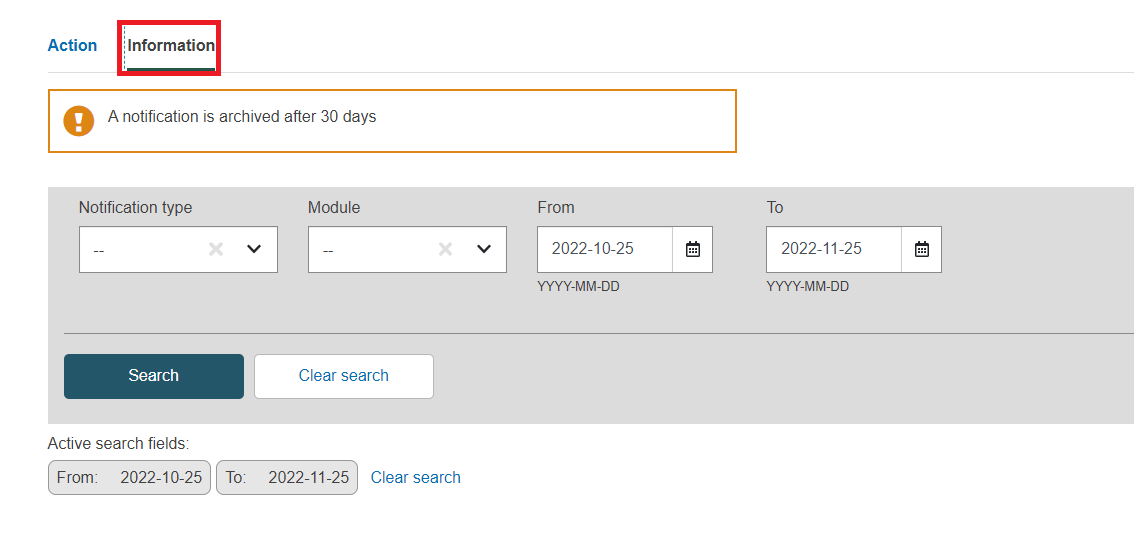
Go to the information section:
Define the fields to refine your search and then click on search.
Note
At least one field must be filled in.
The system will present you the relevant notifications, including the estimated start date (in case of a PMCF/PMPF notification) and the deadline for the CA to react.
Note
The Sponsor can start a PMCF/PMPF without the validation of the CA, because it is a post-market investigation. However, it is mandatory for the Sponsor to notify the CA about the existence of the PMCF/PMPF.
Note
The CA can view all submitted applications, but it can take action only on those for which it is responsible. A CA is responsible for an application if it is selected in the Local Competent Authority field of the National information section.
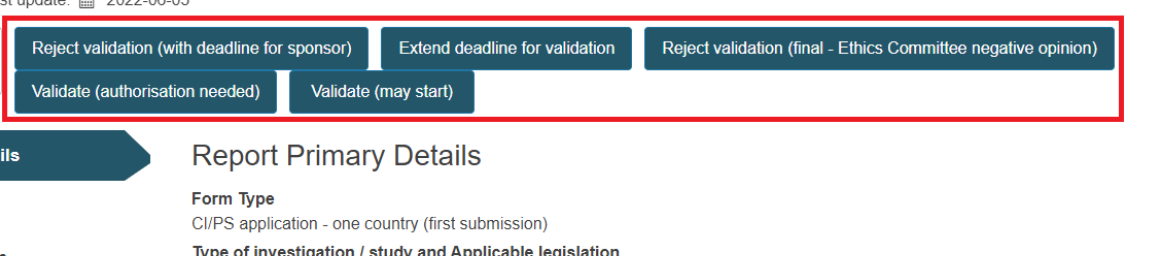
The CA can see at the top of the page the actions that change the state of the application.
Note
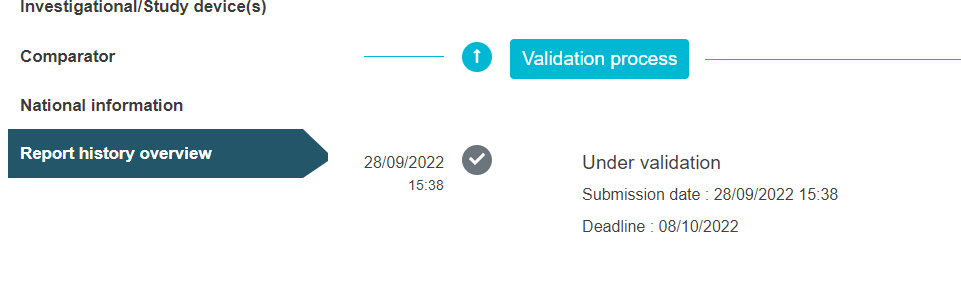
The view below applies to CI/PS application – one country in state under validation.
Under the tab Report history overview you can follow the validation progress of the application.
For more information about the indicative deadlines of the validation process, see chapter Deadlines applying to the CI/PS application – one country.
Extend deadline for validation
Reject validation (with deadline for sponsor)
Reject validation (final - Ethics Committee negative opinion)