Language information
Provide the following information of the CI/PS in the relevant national language:
Full title
Title for lay people
Description for Design methodology – Other – only if this value was selected for Design methodology previously
Description for Type of subjects – Other – only if this value was selected for Type of subjects previously
Primary objective
Secondary objective
Other objective(s)
Primary endpoint
Secondary endpoint
Other endpoint(s)
Overall synopsis
Inclusion criteria
Exclusion criteria
National language-specific Investigational/study (and Comparator) device(s) information sections:
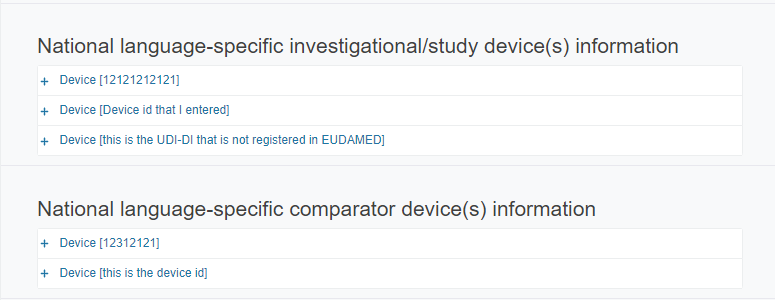
As soon as the sections Investigational/Study devices and Comparator are filled in, the system will create the correct number of sub-sections, if any.
For each sub-section (Investigational/Study device(s) and Comparator), provide the following information in the relevant language:
Device name – not applicable to PMCF/PMPF notification or to the Comparator section
Device trade name – not applicable to PMCF/PMPF notification or to the Comparator section
Device description
Intended (clinical) purpose
Provide the following documents in the national language:
Instruction for use
Informed consent/Patient information leaflet *
Ethics Committee opinion *
Proof of insurance
Other national requirements
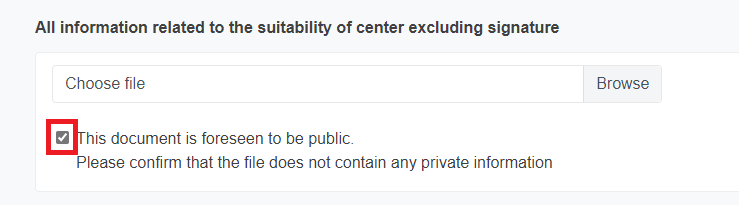
* For these documents you must acknowledge that they do not contain private information, as they are expected to be public. To do it, tick the box.
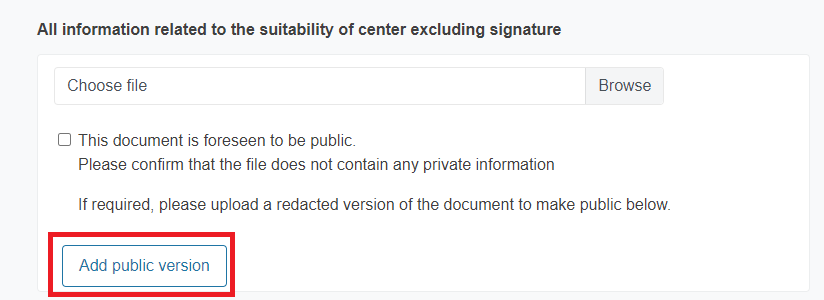
If you are uploading a document that is expected to be public and it contains private information, you must upload a redacted version. To do it, click Add public version.