Investigational/study site(s)
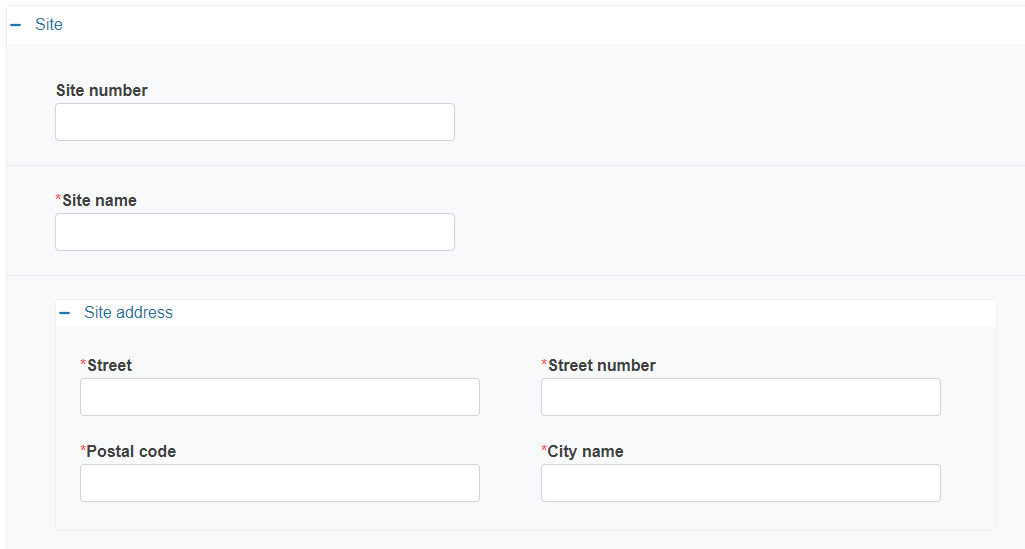
Provide the details of the site:
Site number
Site name
Site address
To add more sites, click Add.
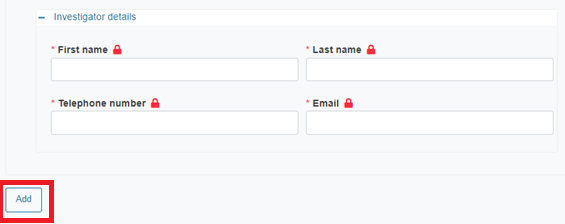
Provide the details of the investigator:
Investigator role
Clinical department
Investigator details (first name, last name, telephone number and email)
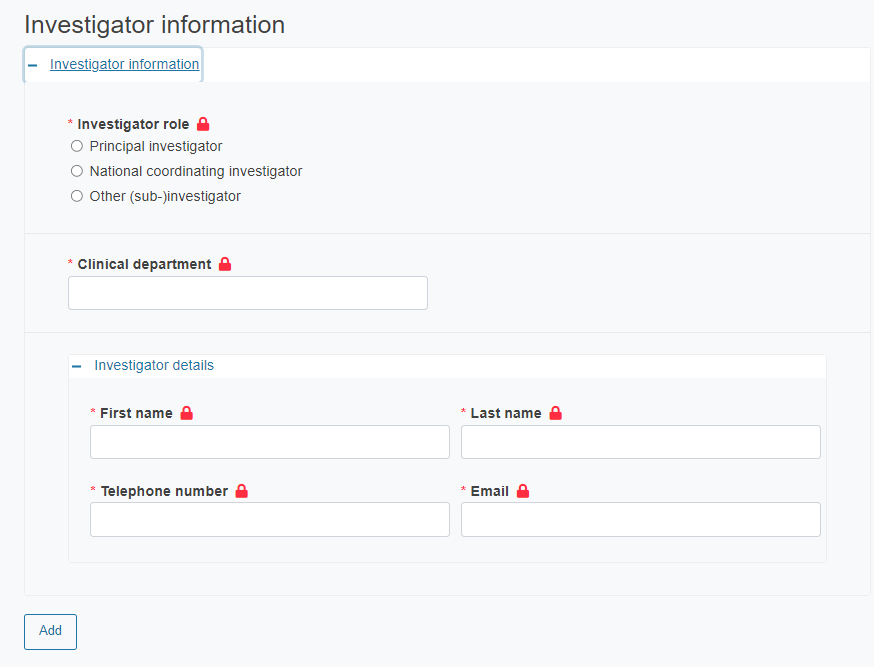
To add more investigators you need to have the Site section expanded. Then click Add.
Provide the Documents related to the investigational/study site:
CV of the investigator
All information related to the suitability of center including signature
All information related to the suitability of center excluding signature *
Other national requirements
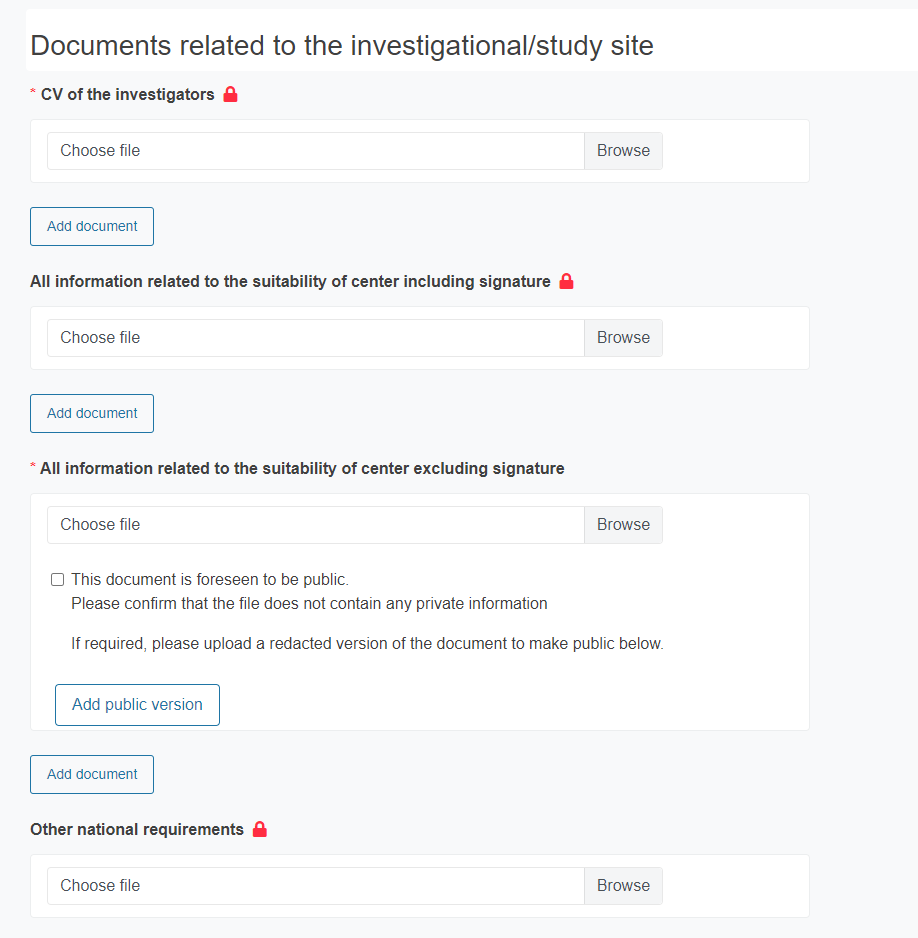
* For this document you must acknowledge that it does not contain private information, as it is expected to be public. To do it, tick the box.
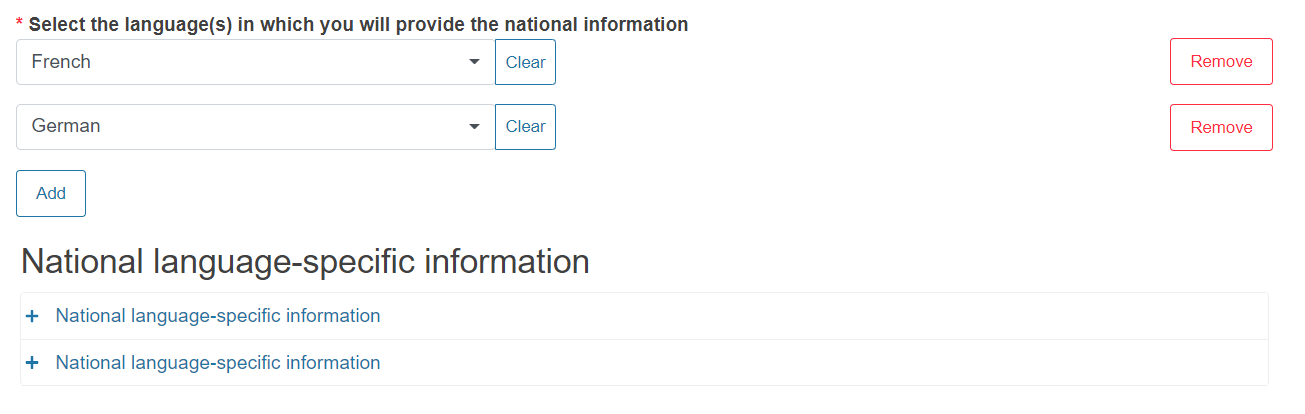
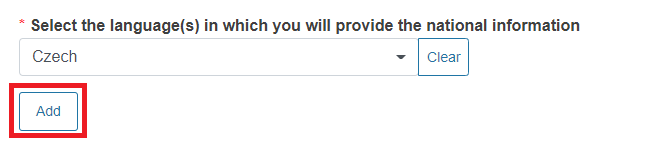
Select the language(s) in which you will provide the national information from the dropdown list.
Note
The system filters the national language(s) of the country of the application.
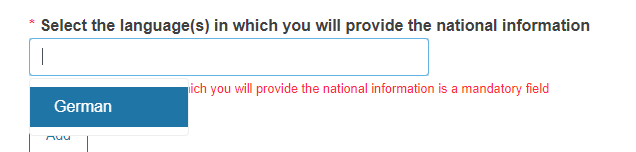
You can add more languages by clicking Add.
As soon as a language is defined in the field above, the system will generate the relevant number of dedicated National language-specific information sections: