How to complete the application
Note
For this guide we are using the CI/PS application - one country form. The other forms available are very similar but we will identify their differences when relevant.
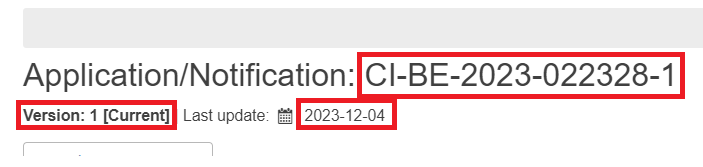
Once you have filled in all the fields as explained in the previous chapter, the system will create a draft record and generate an Application ID, the EU SIN and the National SIN.
The Application ID is different for every application or notification and has the following format:
For CI/PS: CI or PS (identifies if it is a clinical investigation or a performance study). For PMCF/PMPF: CF or PF (identifies if it is a post-market clinical follow up or a post-market performance follow up).
The country code or ‘EU’ if it is an application for coordinated assessment
The year of initiation of the application/notification
Six-digit number that resets every year. There will be a new series of unique identifiers every year, composed by the year and the six-digit number. This composed identifier is unique in the entire system, it is not repeated per country or per type of investigation/study or per sponsor.
Application/notification sequence number (in the example CI-BE-2023-022328-1, -1 means that this is the first submission for the given clinical investigation application; in the example CI-AU-2022-000555-2, -2 means that there is a submission for an additional country (Austria) for the same clinical investigation; in the example CF-BE-2023-001119-R2, -R2 means that two resubmissions were done for the same application)
The EU SIN is the unique identifier of the investigation/study and will be shared among all resubmissions and submissions for additional country(-ies) that refer to the same first submission. It is built as follows:
The National SIN, which is displayed in the National information section, is the same as the EU SIN except for the code ‘EU’ which is replaced with the relevant country code.
element 1 above
EU
elements 3 and 4 above
At the top of the page you can see the version number of the draft record and the date of the last update.
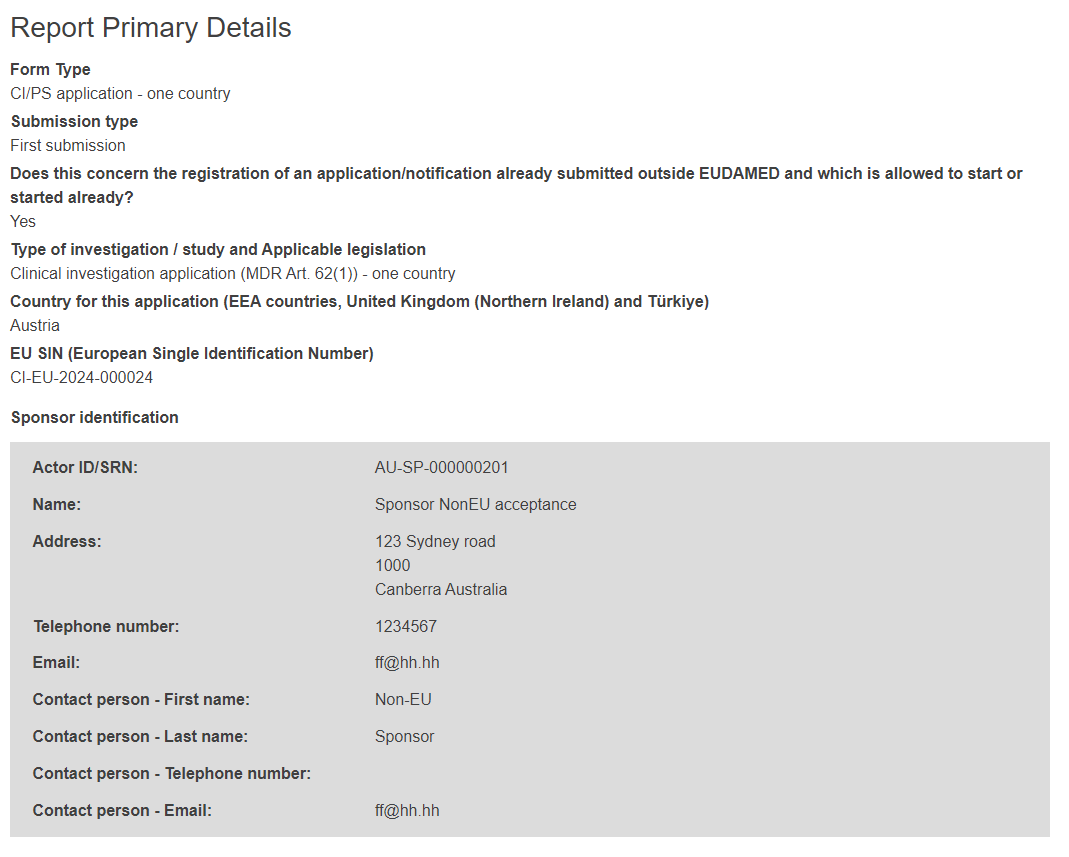
The first section of the form, named Report Primary Details, gives you an overview of the data already entered.
Note
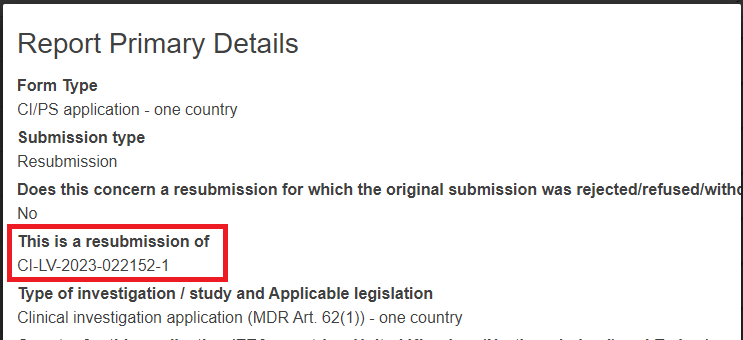
If you are resubmitting an application, the report primary details will have an extra field: This is a resubmission of which displays the original Application ID for which the current form is a resubmission.
If you are submitting a pre-existing application/notification the report primary details will have the following fields:

Common documents and statements