Clinical investigation study
Important
If you are submitting an application for additional country(ies) or a pre-existing application/notification for which an EU SIN exists, this section is not pre-populated and you need to fill in the national information for each additional country(ies).
At the top of the screen, every national information section (one per country, in case of an application for coordinated assessment), you can see the national single identification number (SIN).

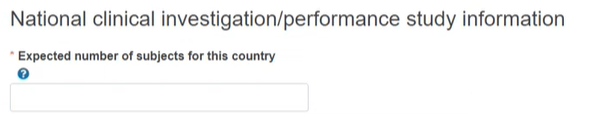
Indicate the expected number of subjects for the country.
Reply Yes or No to the question Is the sponsor commercial?
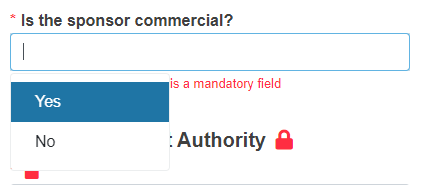
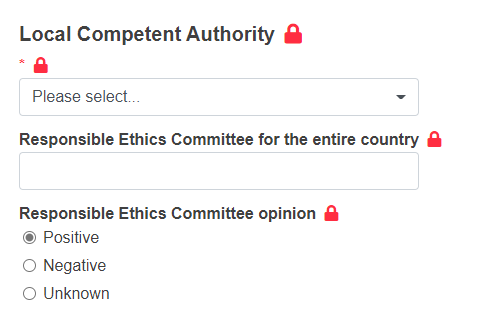
Provide the details of the Local Competent Authority:
Identification of the Local Competent Authority (the system will give you the relevant results) - this value determines the Competent Authority that will validate and authorise (when relevant) the submission
Responsible Ethics Committee for the entire country
Responsible Ethics Committee opinion (define if is Positive, Negative or Unknown).