Device information
Click on Device information from the menu on the left:
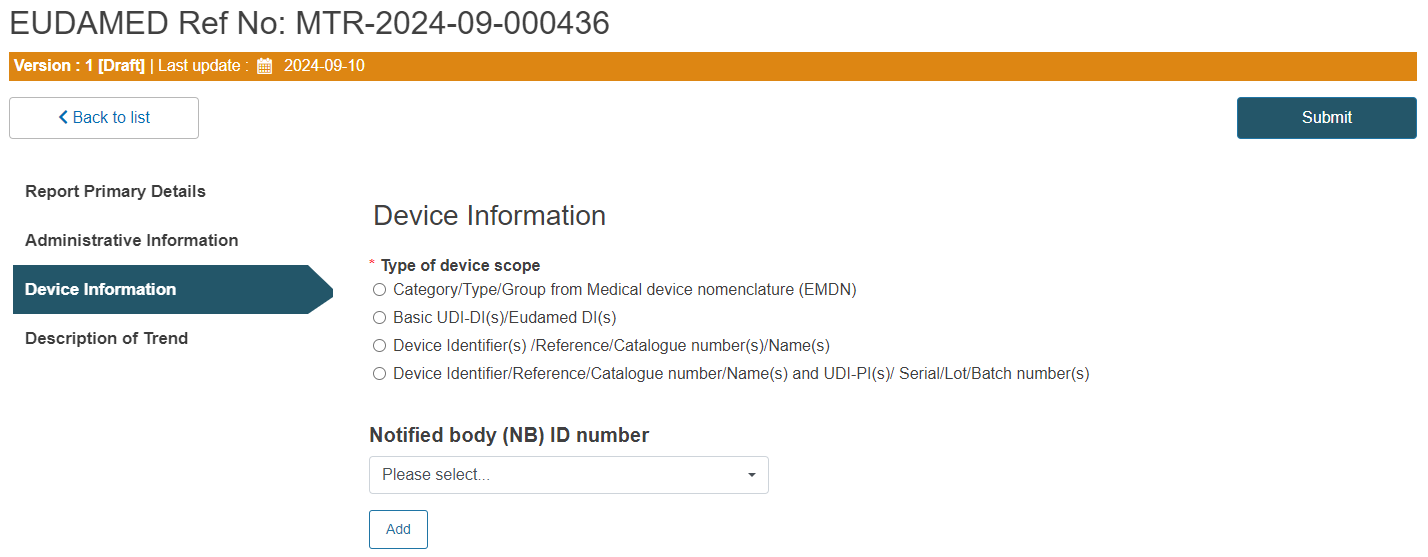
Select the type of the device scope.
Tip
Depending on the device scope type selected, the process will slightly vary, as demonstrated in each of the four options below.
Follow Option A, B, C or D:
A. Steps for Category/Type/Group from Medical device nomenclature ( EMDN):
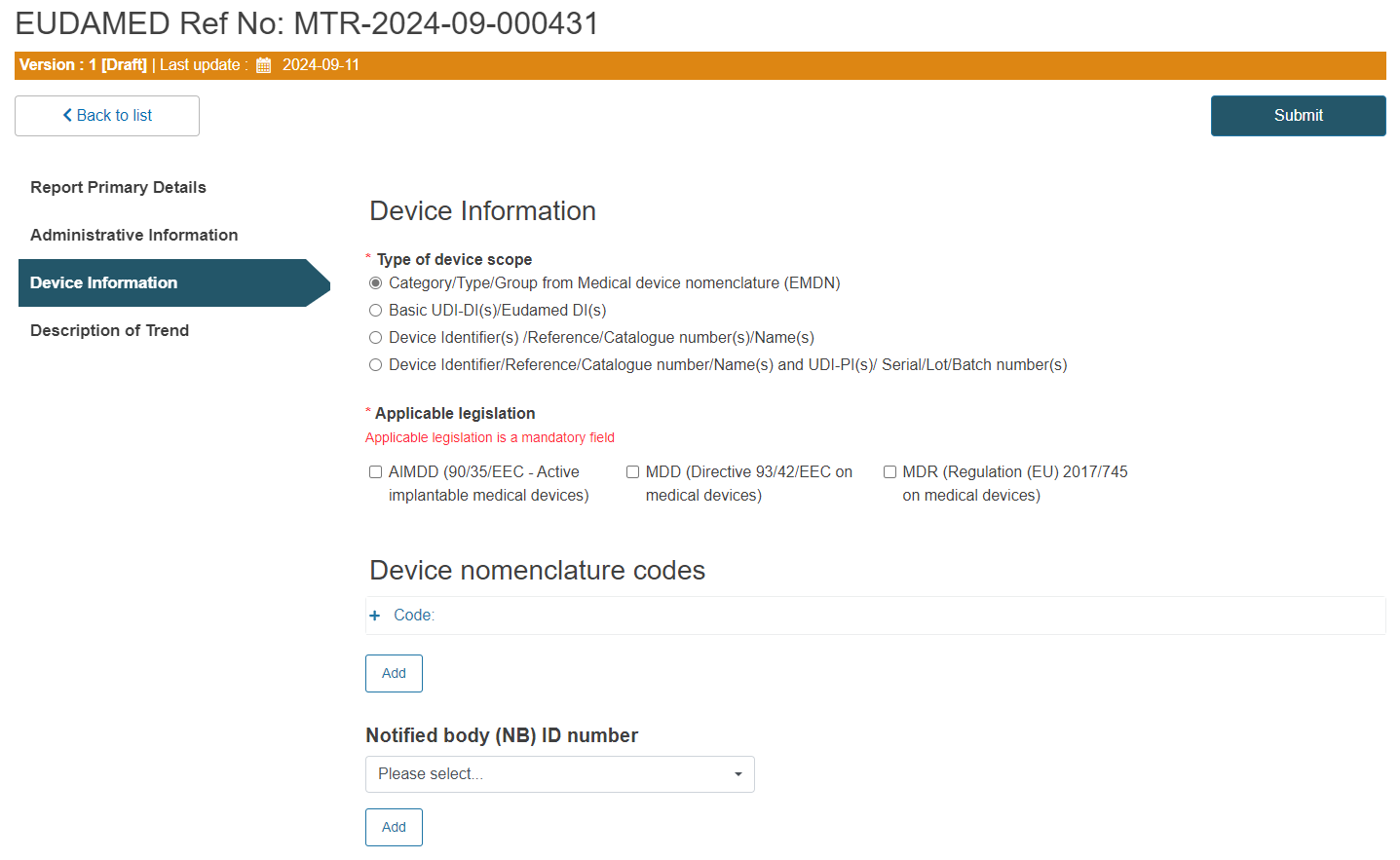
Select the applicable legislation:

Click on the plus sign next to Code to enter the device nomenclature code:
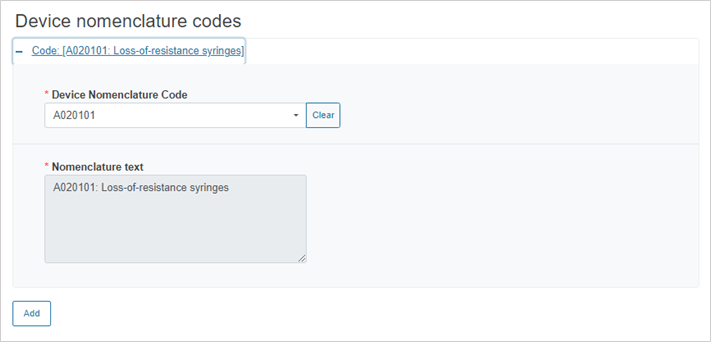
To add more nomenclature codes, click on Add.
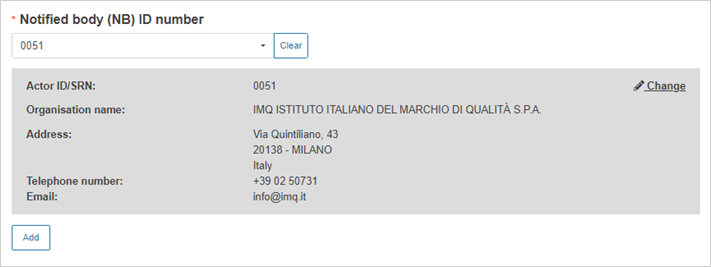
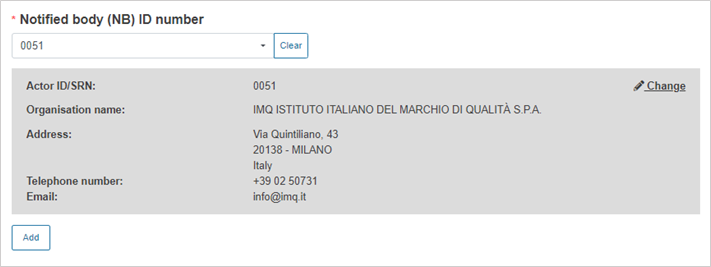
Select the Notified Body ID number:
B. Steps for Basic UDI-DI(s)/EUDAMED DI(s):
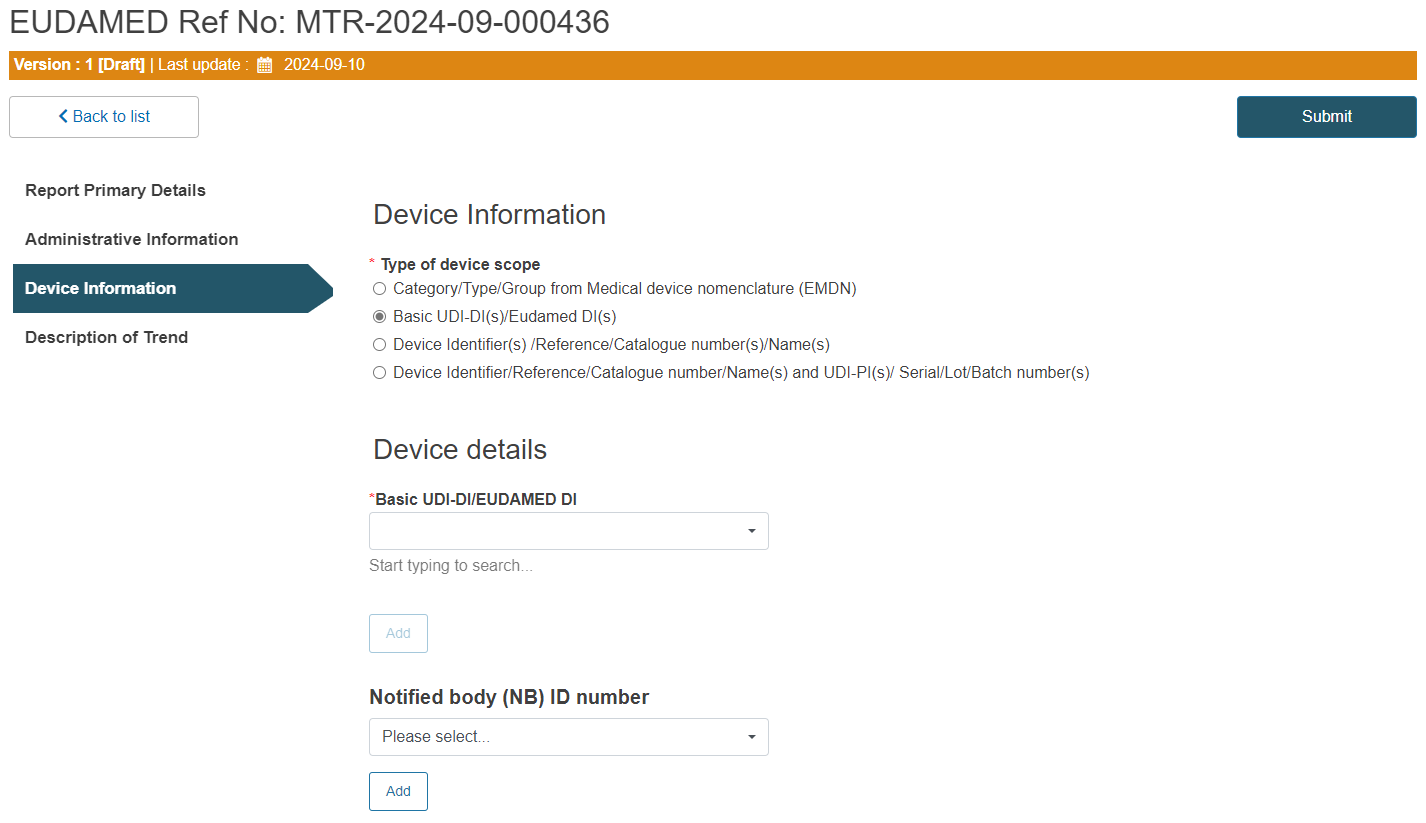
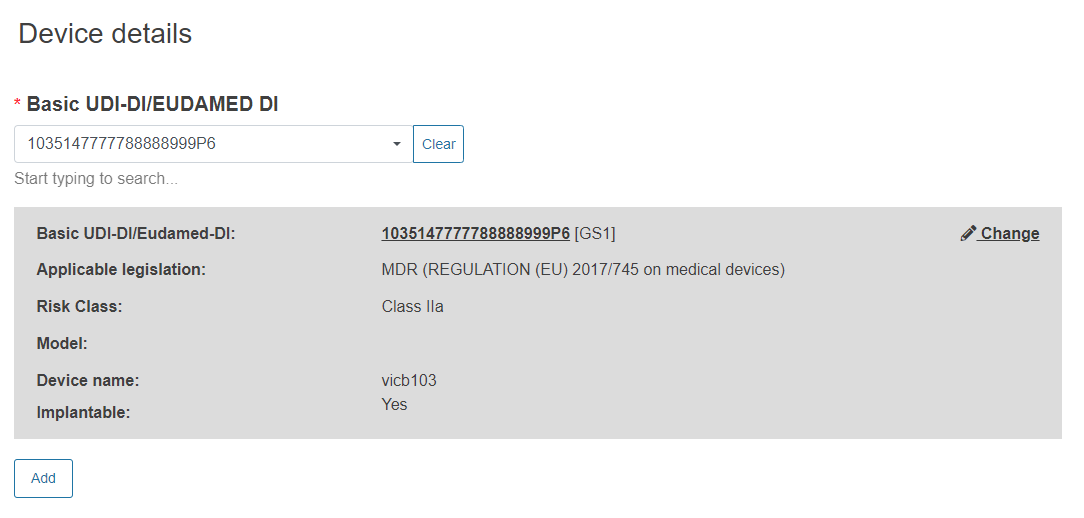
Enter the Basic UDI-DI/EUDAMED DI:
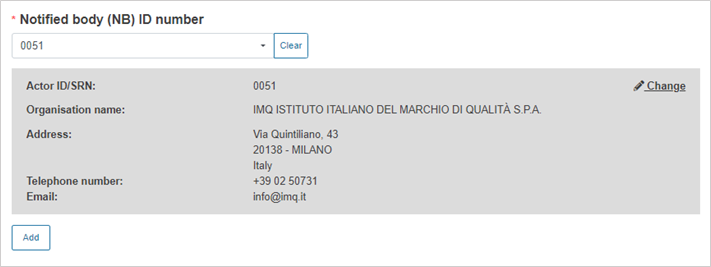
Select the Notified Body ID number:
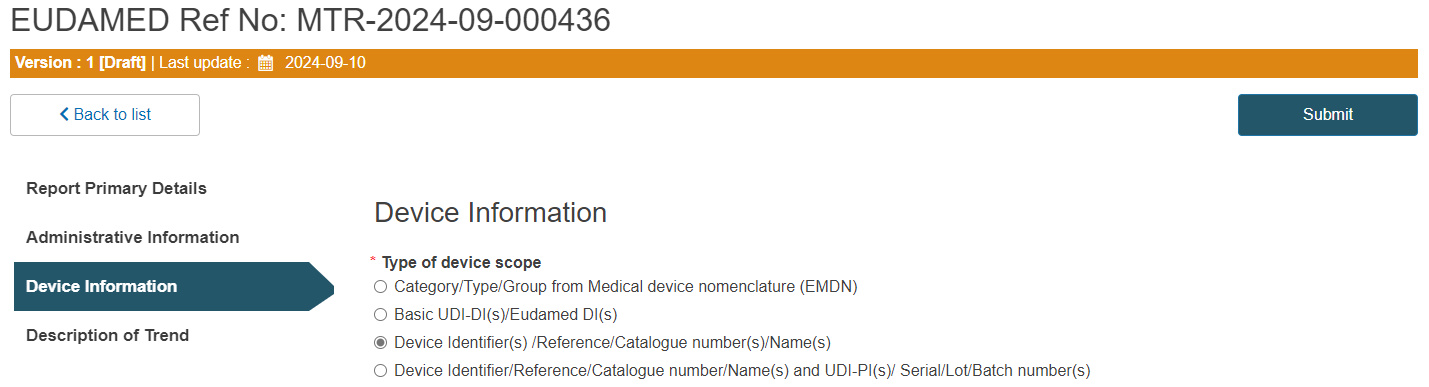
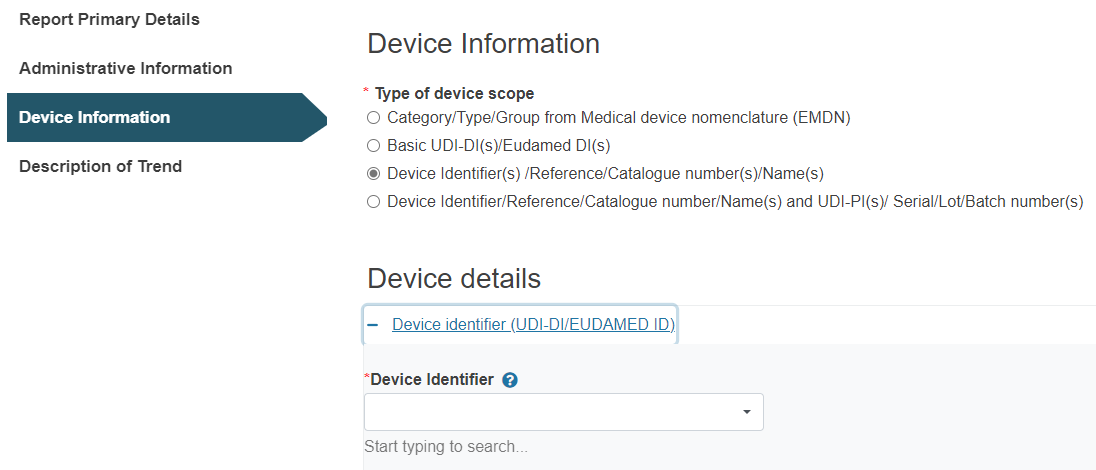
C. Steps for Device Identifier(s) /Reference/Catalogue number(s)/Name(s):
Click on the plus sign next to Device identifier and type it in:
Note
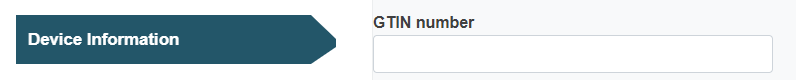
In case of a special device type (requiring a Master UDI-DI), the GTIN field will appear for you to fill in:
Select the Notified Body ID number:
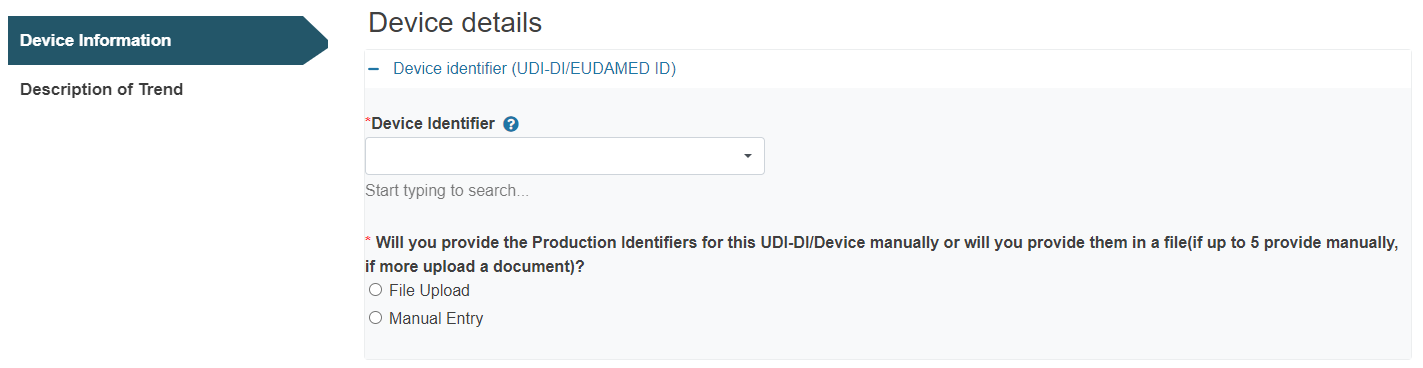
D. Device Identifier/Reference/Catalogue number/Name(s) and UDI-PI(s)/ Serial/Lot/Batch number(s):
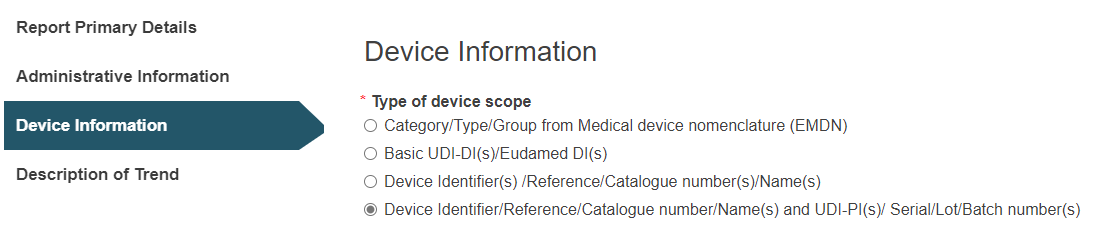
Click on the plus sign next to Device identifier to select it:
Note
In case of a special device type (requiring a Master UDI-DI), the GTIN field will appear for you to fill in:

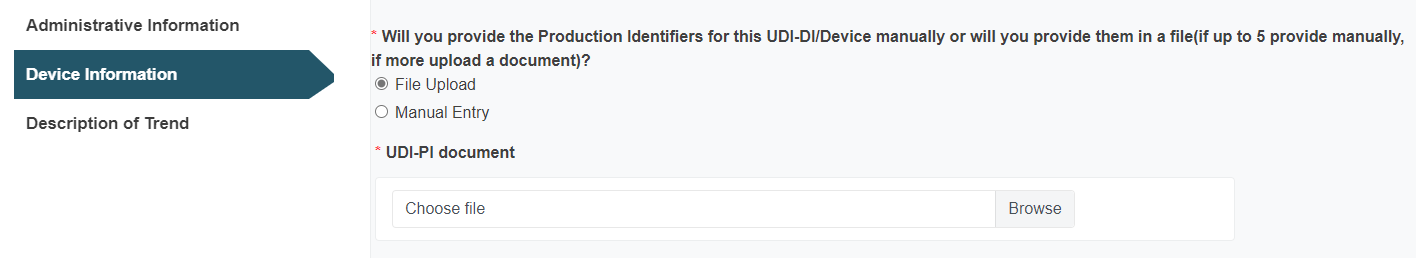
Click Browse to upload a UDI-PI file:
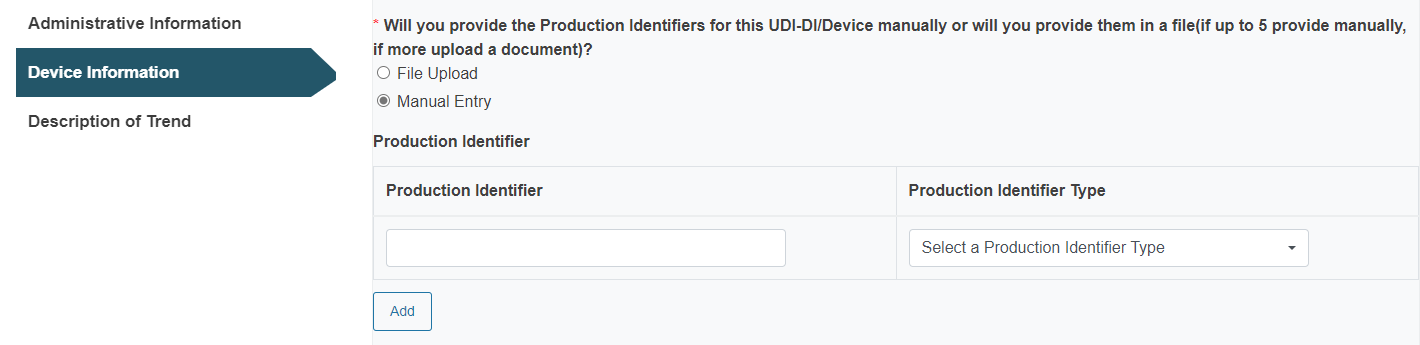
Alternatively, enter the UDI-PI manually:
Select the Notified Body ID number:
