Administrative information
Click on Administrative Information from the menu on the left to access the section:
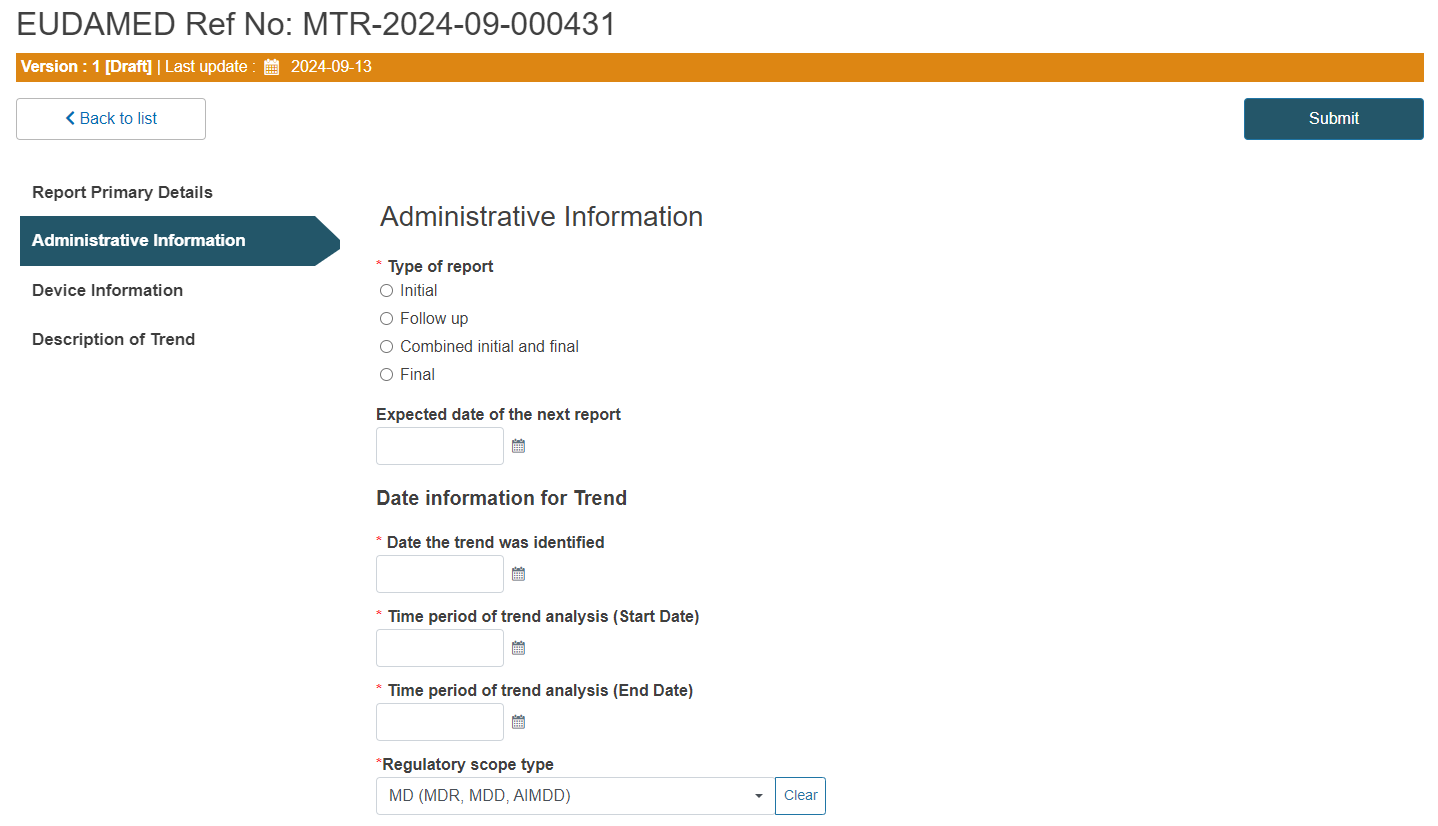
Choose the type of the report:

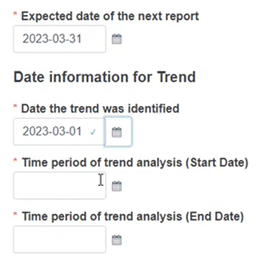
Fill in the expected date of the next report and all the relevant dates:
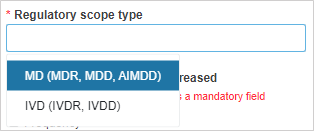
Select the applicable legislation:
Choose the criteria forming the basis of the report:

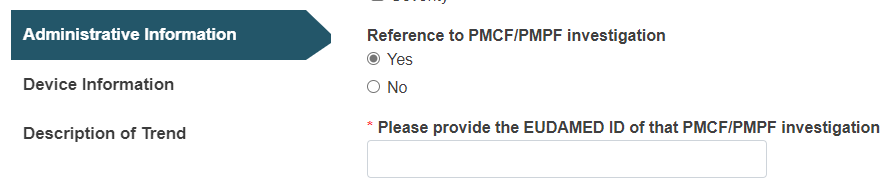
If applicable, provide the PMCF/PMPF EUDAMED ID reference to a PMCF/PMPF investigation:
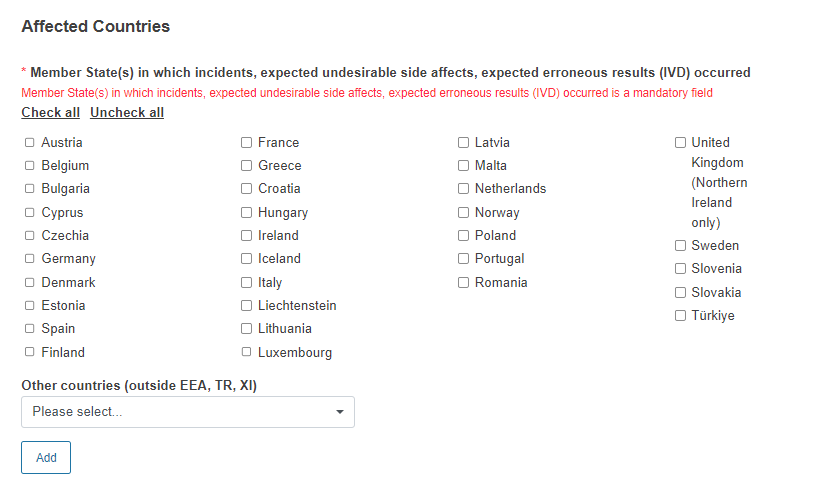
Select the affected country(-ies):
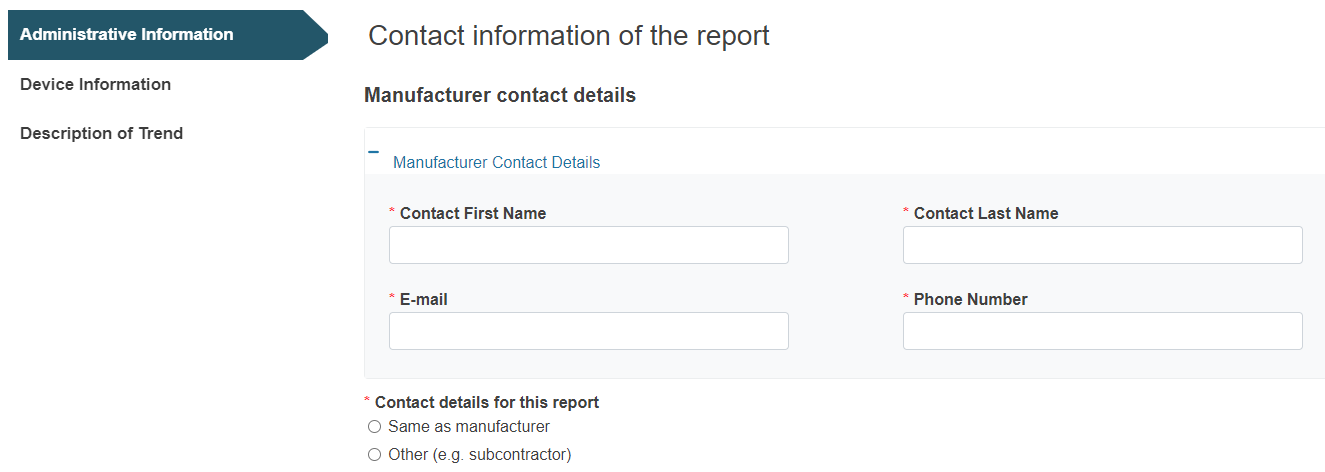
Provide the contact details of the Manufacturer (or Authorised Representative) and the Submitter for this report: