Device information
Reply Yes or No to the question Do any of the following device types apply?
If you reply Yes, select the Device type(s):

Reply to the question Is the device CE marked?
Based on the response, certain fields will become available (and mandatory) or not:
Is the device CE marked?
Yes
Is the device registered in EUDAMED?
Yes
Provide:
Unique Device Identification (UDI-DI / EUDAMED ID)
Confirm that the CE-marked device will be used outside the scope of its CE mark by ticking the box
No
Provide:
UDI-DI - not registered in EUDAMED
Issuing entity
Confirm that the CE-marked device will be used outside the scope of its CE mark by ticking the box
No
No further action
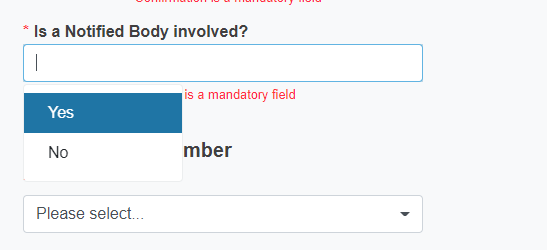
Reply Yes or No to the question Is a Notified Body involved?
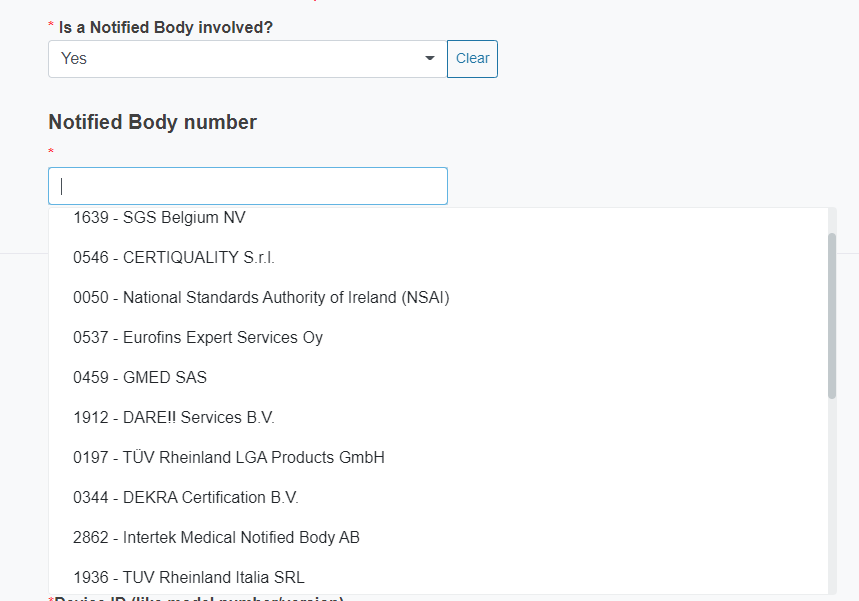
If you reply Yes, you must select from the drop-down list the Notified Body number.
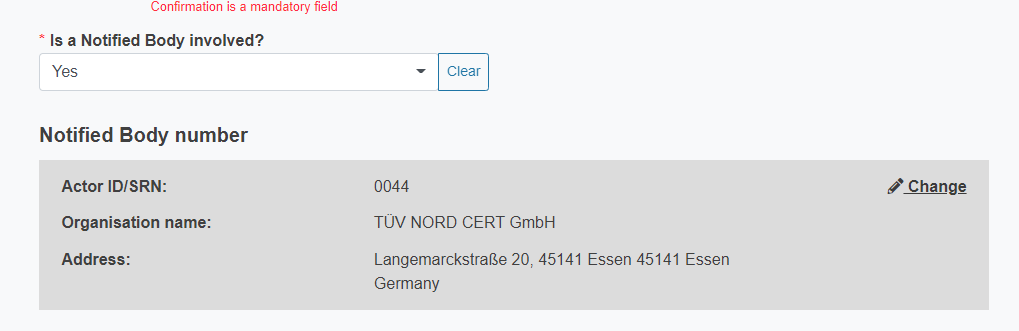
Once you select the Notified Body number, its data will be displayed.
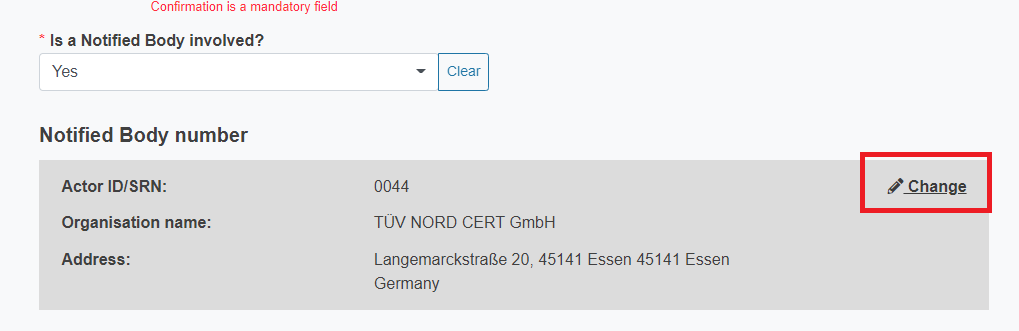
To change the Notified Body number click Change at the top right of the grey box.