Device details
In this section provide the details of the device:
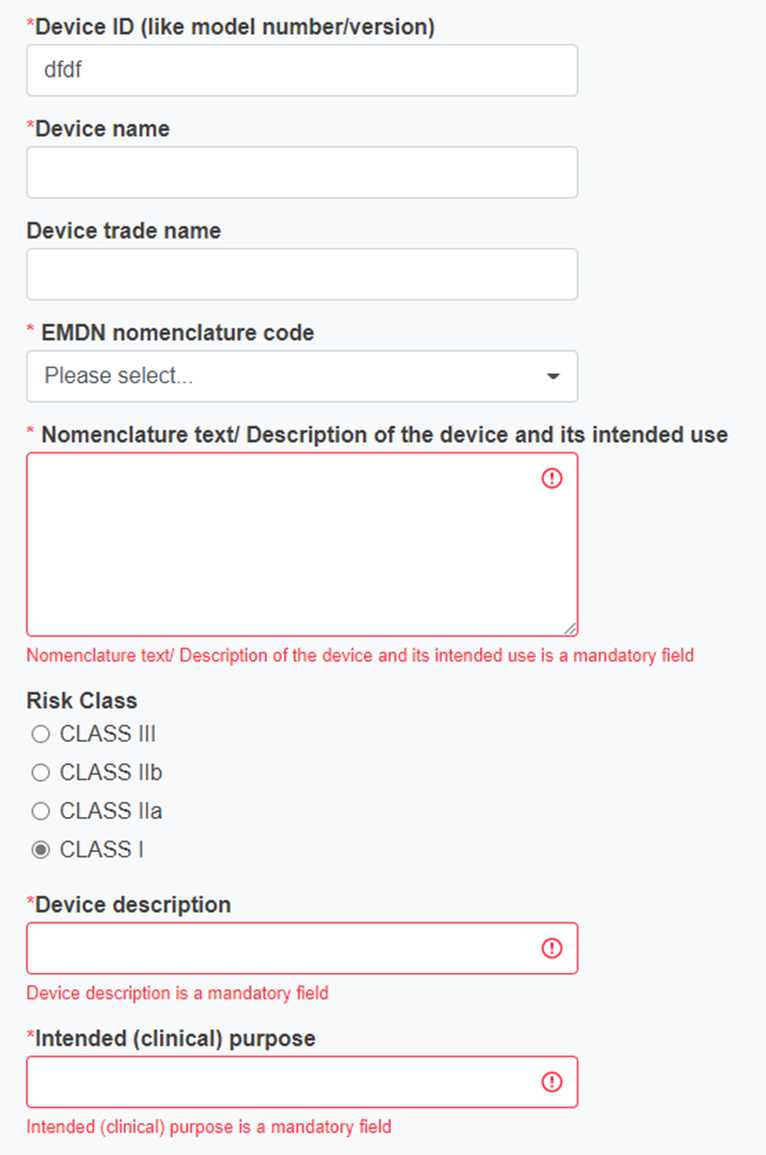
Device ID
Device name
Device trade name
EMDN nomenclature code
Nomenclature text/ Description of the device and its intended use – retrieved automatically based on the code selected
Risk class (choose from the list – only one option is possible)
Device description
Intended (clinical) purpose
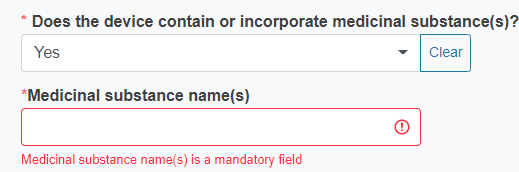
Reply Yes or No to the question Does the device contain or incorporate medicinal substance(s)?
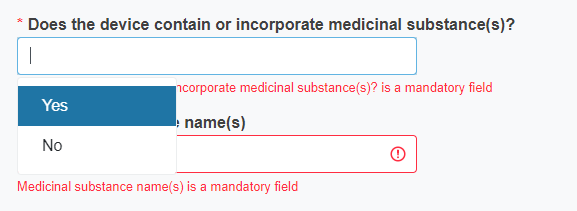
If you reply Yes, you must provide the name of the medicinal substance.
To add more medicinal substances click Add.
Reply Yes or No to the question Does the device include human blood or plasma derivatives?
Reply Yes or No to the question Does the device incorporate, as an integral part, or is it manufactured using non-viable biological substances?
If you reply Yes, you must complete the relevant value(s) for the field Please select the appropriate value(s).
You can select several options.

Reply Yes or No to the question Has the device been subject to scientific views/an opinion from an Expert Panel and/or EURL?