Register a CI/PS application
 VIDEO: Resubmission of the CI/PS application/notification
VIDEO: Resubmission of the CI/PS application/notification
 VIDEO: CI/PS Submission for additional countries
VIDEO: CI/PS Submission for additional countries
Note
The system saves the data you enter automatically. There is no Save button.
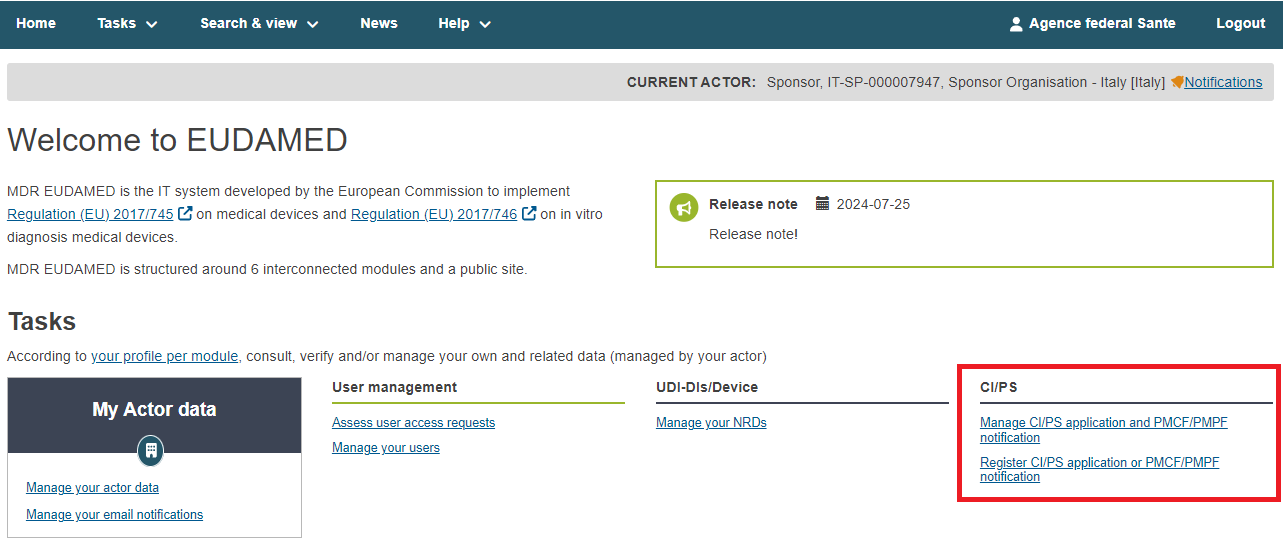
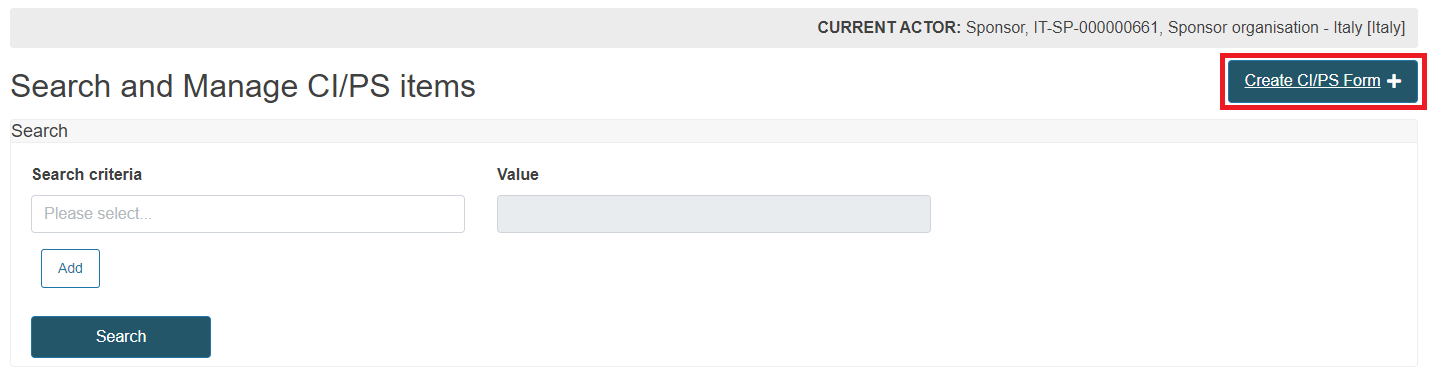
To start, you have two options in the dashboard:
Click Register CI/PS application or PMCF/PMPF notification
Click Search and Manage application/notification and click the Create CI/PS Form button at the top right corner.
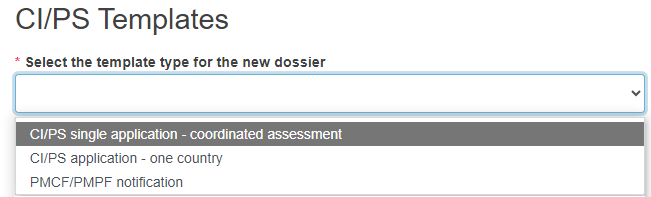
Select the form you want to submit from the drop-down list.
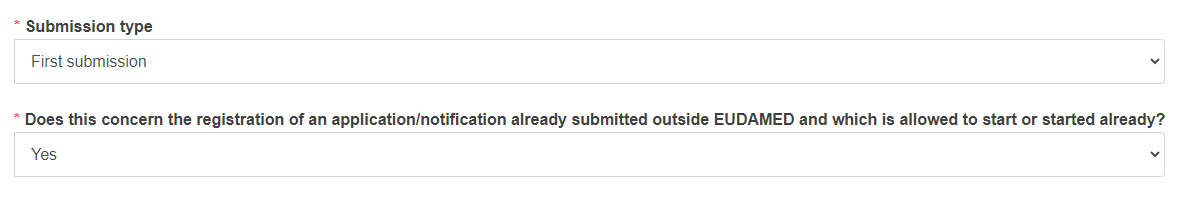
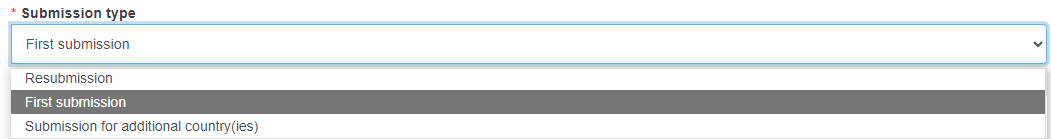
Select the Submission Type from the drop-down list.
Submission for additional country(ies):
If you select CI/PS application – one country in the field Select the template type for the new dossier, this means that the new application will be submitted only to the country which will be selected in step 7 below.
If you select CI/PS single application – coordinated assessment, this means that the new application will be submitted to all countries specified in the dedicated field Country for this application in the Coordinated assessment information screen.
If you select PMCF/PMPF notification, this means that the new notification will be submitted only to the country which will be selected in step 7 below.
When you create the application for submission to additional country(ies), it will be populated with the information from the latest application (version) that was submitted for that EU SIN. If all the information corresponds with the new submission, you only need to fill in the national information for each new country.
All the fields are editable, except the CI/PS plan code (see support clip CI/PS Submission for additional countries).
Resubmission:
When you create a resubmission, the form will be populated with the information from the original application. You need to adapt the data as appropriate before submitting the new application/notification.
All the fields are editable, except the CI/PS plan code (see support clip CI/PS Resubmission).
If you are initiating a first submission in EUDAMED of a CI/PS application – one country or a PMCF/PMPF notification: Reply Yes or No to the question Does this concern the registration of an application/notification already submitted outside EUDAMED and which is allowed to start or started already?
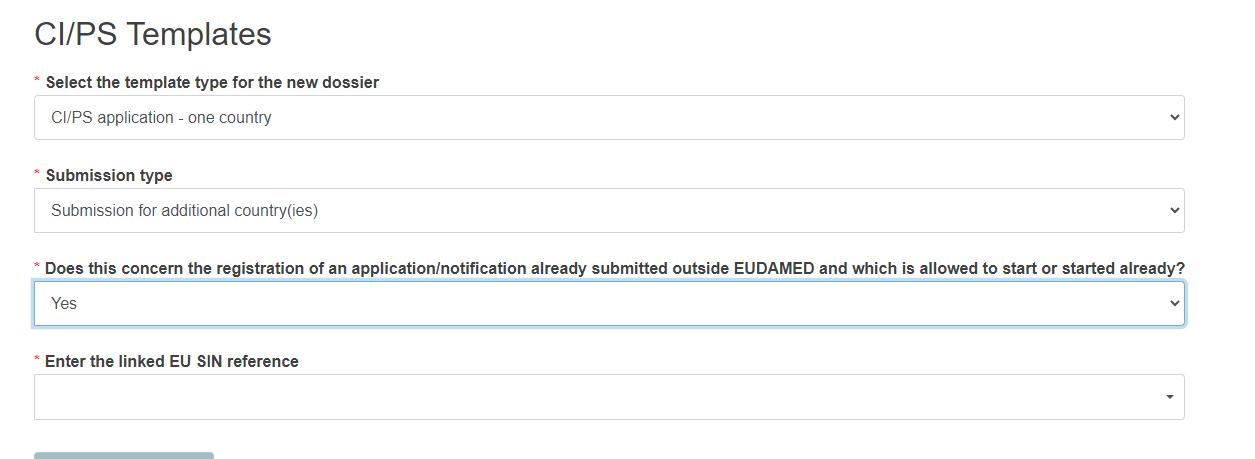
If you are initiating a submission for additional country(ies):
Reply Yes or No to the question Does this concern the registration of an application/notification already submitted outside EUDAMED and which is allowed to start or started already?
Reply Yes or No and enter the linked EU SIN reference, by choosing the relevant result from the drop-down list.
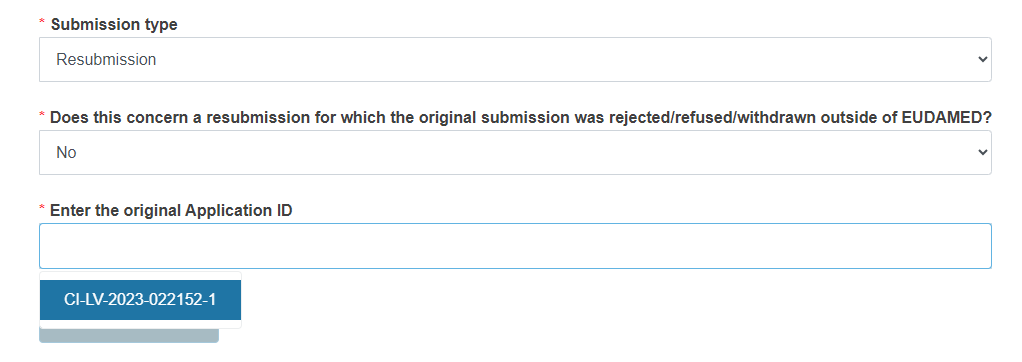
If you are resubmitting an application/notification:
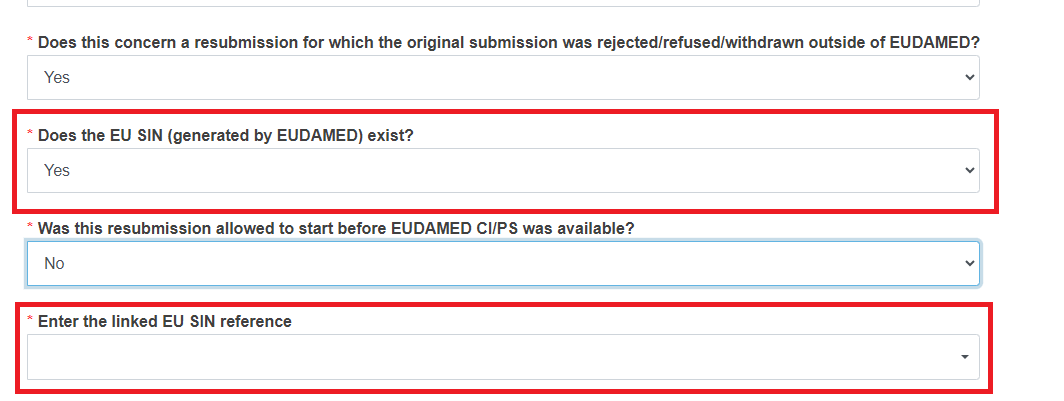
Reply Yes or No to the question Does this concern a resubmission for which the original submission was rejected/refused/withdrawn outside of EUDAMED?
If you reply No, enter the original application ID by choosing the relevant result from the drop-down list.
The system will retrieve all the applications/notifications that have been withdrawn by your actor and applications submitted by your actor that have been rejected or refused by the Competent Authority.
If you reply Yes to the previous question, reply Yes or No to Does the EU SIN (generated by EUDAMED) exist?
Reply Yes or No to the question Was this resubmission allowed to start before EUDAMED CI/PS was available?
If you replied Yes to the question Does the EU SIN (generated by EUDAMED) exist?, you will need to select a value for Enter the linked EU SIN reference:
Select the proposed Actor ID/SRN.
In the case where this is a first submission or any submission for which no related EU SIN exists, you need to select the type of investigation/study from the drop-down list.
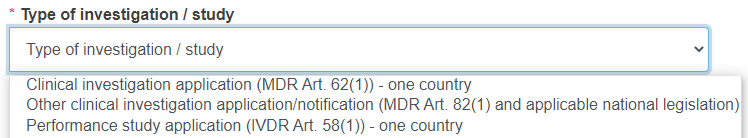
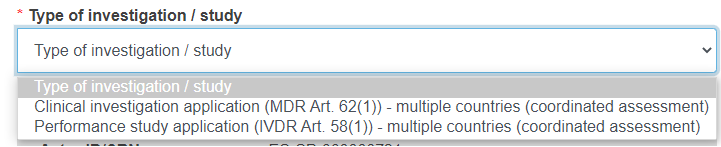
The above view depends on the form you are submitting.
For a PMCF/PMPF notification (first submission) you will have the following options:

For a CI/PS single application – coordinated assessment (first submission) you will have the following options:
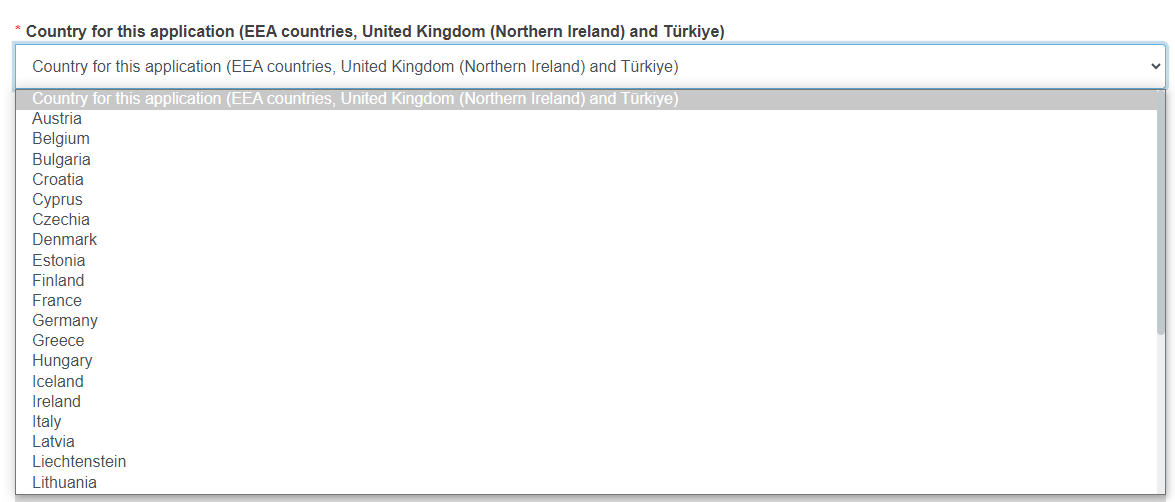
Select the country from the drop-down list. Only EU+ [19] countries are available for selection. This step does not apply to the form type CI/PS single application – coordinated assessment or to the Resubmission that exists in EUDAMED.
Important
If you are initiating a submission for additional country(ies), you cannot submit an application/notification for a country for which an application/notification already exists on the same EU SIN.
Click Create Form at the bottom of the screen.
Below you can find a table with a summary of the fields that you have to fill in, depending if you are managing a first submission, a resubmission or a submission of additional country(ies):
Submission type | Type of investigation/study and Applicable legislation | Country for this application | Enter the original Application ID | Enter the linked EU SIN reference | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
First submission | Yes | Yes[a] | |||||||||||||||||||||||||||||||||||||||||||||||
Resubmission | Yes | ||||||||||||||||||||||||||||||||||||||||||||||||
Submission for additional country(ies) | Yes[b] | Yes | |||||||||||||||||||||||||||||||||||||||||||||||
[a] Except for CI/PS single application – coordinated assessment. [b] Except for CI/PS single application – coordinated assessment. | |||||||||||||||||||||||||||||||||||||||||||||||||