Study device scope
Choose the device purposes:

Reply Yes or No to the question Is this a combined investigation/study testing two different products (i.e. an MD and an IVD, which in combination reach an intended purpose) and/or is it an investigation/study testing a combined product (MD + IVD)?
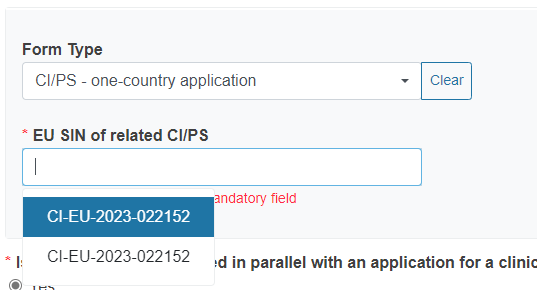
If you reply Yes, a new section will appear, from where you can select the form type you want to link to the application/notification. Then choose from the drop-down list the EU SIN of the related CI/PS application or PMCF/PMPF notification. The drop-down list only displays forms owned by the current Sponsor.

Important
You can only link forms that have been created by your Sponsor actor. You cannot link draft forms.
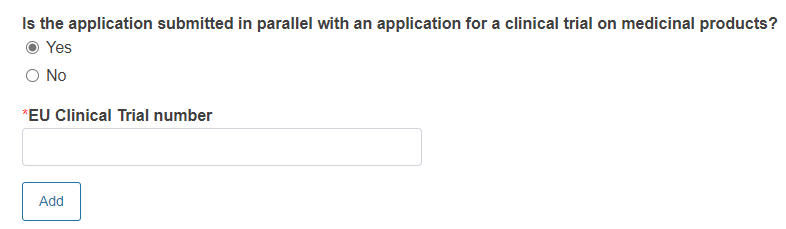
Reply Yes or No to the question Is the application submitted in parallel with an application for a clinical trial on medicinal products?
If you reply Yes, you must provide EU Clinical Trial number.
To add other EU clinical trial number click Add.