Population
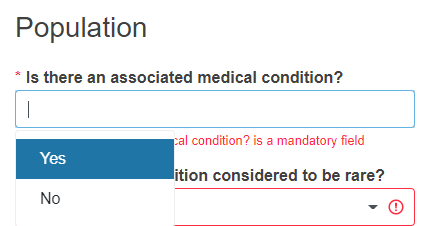
Reply Yes or No to the question Is there an associated medical condition?
If you reply Yes, the question Is the medical condition considered to be rare? will appear for you to reply.
For MDR - Select the therapeutic area by clicking + Therapeutic area.
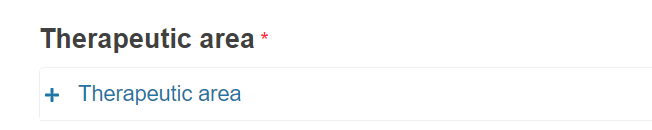
A new section will appear for you to choose the medical and therapeutic areas.
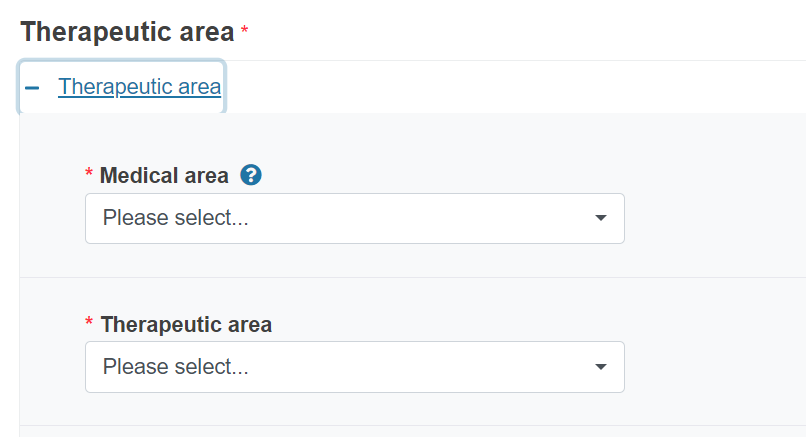
The medical area you choose will determine the options you will have under the therapeutic drop-down list.
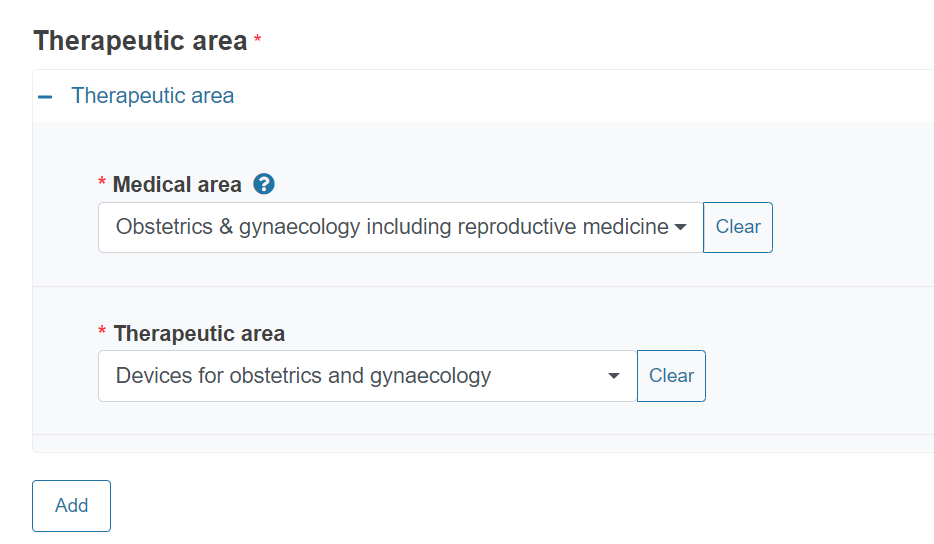
You can add more therapeutic areas by clicking Add.
Note
In case of Performance Studies (IVDR) the section Diagnostic area will show instead of Therapeutic area.
A new field will appear to select the Diagnostic area.
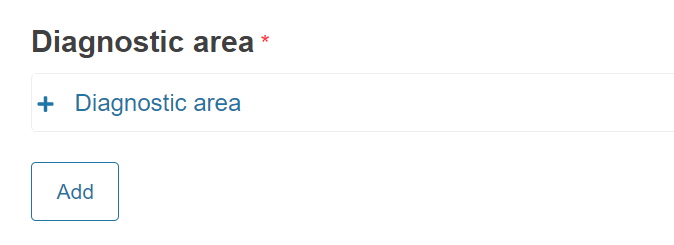
The Diagnostic area codes are the EMDN nomenclature codes for IVD devices. The values are restricted to the EMDN codes starting with “W” (In vitro diagnostic medical devices) at level 3 or 4 of the nomenclature structure. See the European Medical Device Nomenclature (EMDN) for more information.
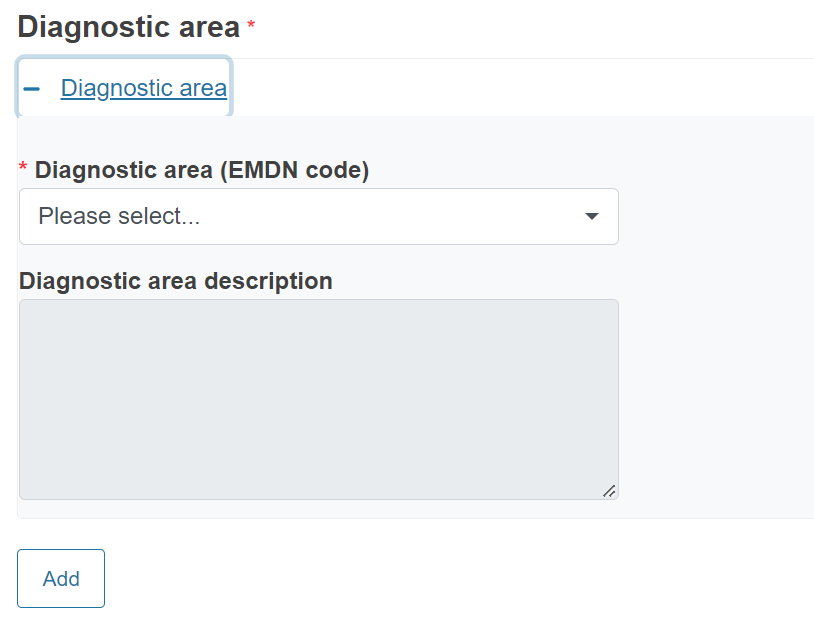
To complete the field and retrieve the description, enter “W” and at least the first 4 digits of the nomenclature code.
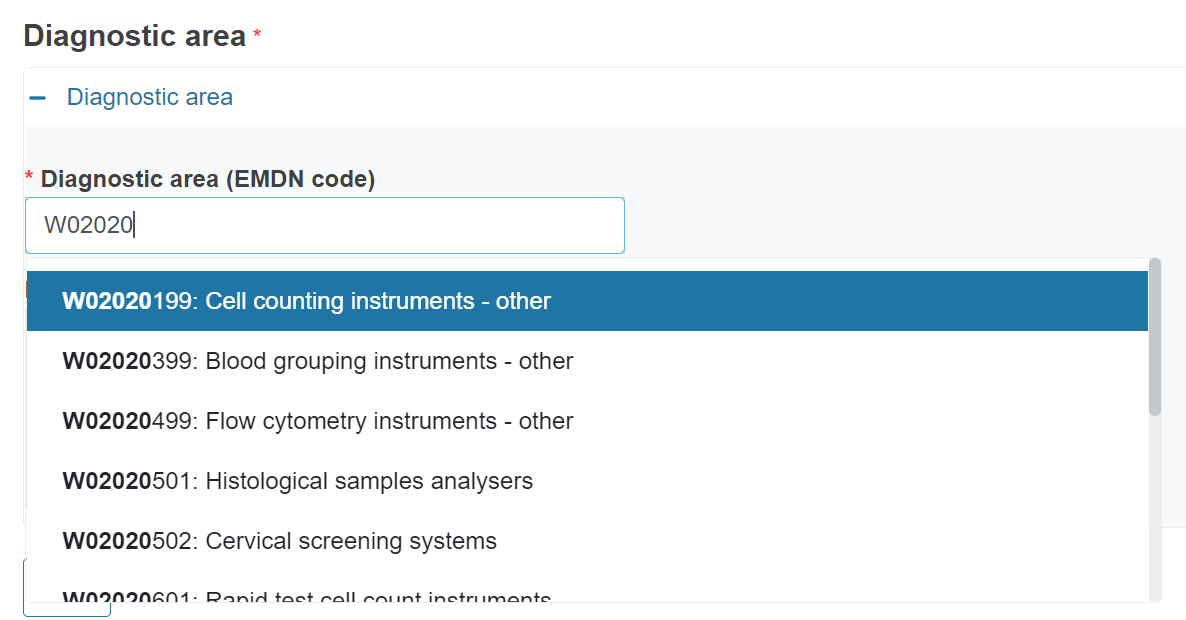
The system will display the relevant options to select from.
You can add several Diagnostic area codes by clicking Add.
Provide the sex of the subjects.

Indicate the inclusion and exclusion criteria.
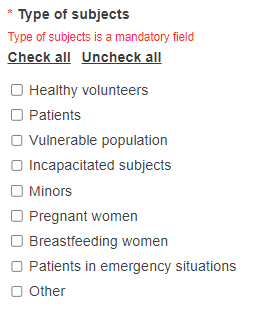
Choose the type of subjects.
Choose the age range.