CECP Registration
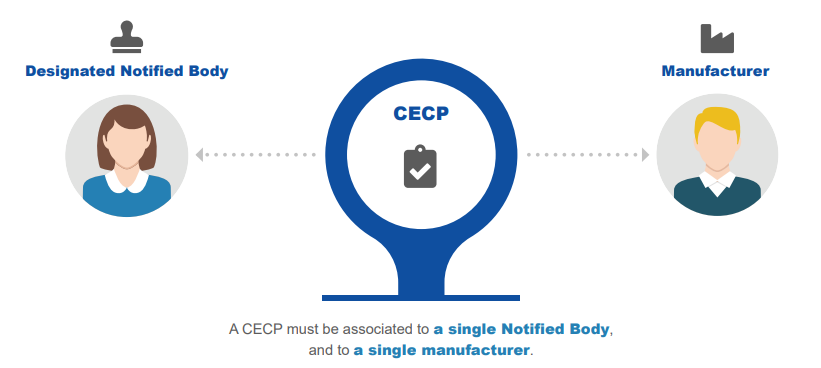
When issuing certificates for class III implantable devices and class IIb, the mechanism for scrutiny applies. This is split into two parts where each part deals with certain aspects as defined by the legislation. The first part consists of registering a Clinical Evaluation consultation Procedure (CECP).
 INFOGRAPHIC: Following the CECP procedure
INFOGRAPHIC: Following the CECP procedure
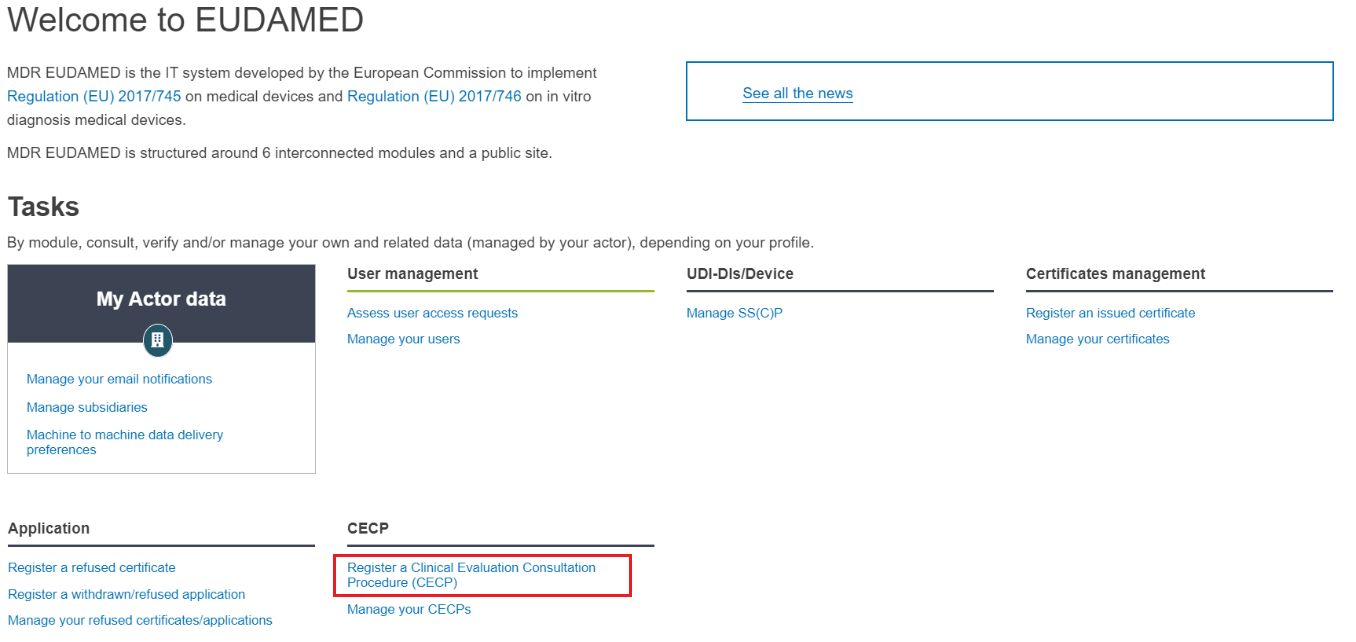
Look for the CECP section at the bottom and click on Register a Clinical Evaluation Consultation Procedure (CECP). You will be brought to the first step of the process:
 |