Common documents and statements
Click Common documents and statements on the left menu.
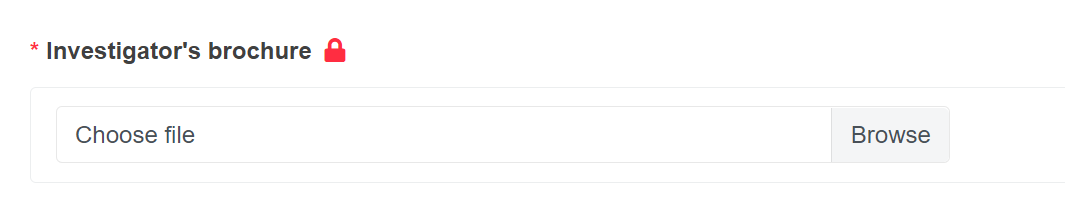
To upload a document, click Browse and then select it from your computer.
Note
All the documents must be uploaded in PDF format.
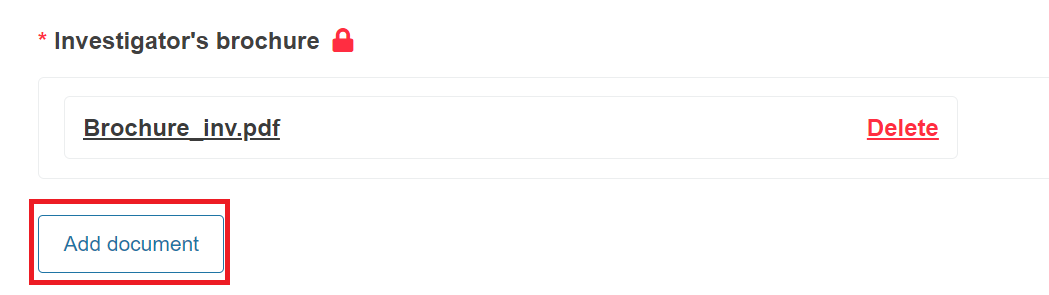
You can add several documents for each topic. To do it, click Add document.
Note
The investigator’s brochure is not mandatory if you are submitting a PMCF/PMPF notification.
Note
Click delete to replace the uploaded file with another one, or remove to eliminate the document instance (only available when there are at least two file instances).
The following documents will not be publicly available:
Investigator's brochure
Clinical investigation/Performance study plan (for CI/PS application only)
PMCF/PMPF investigation plan (for PMCF/PMPF only)
Technical documentation as risk analysis, test report, etc.
Overall synopsis of clinical investigation/performance study (Referenced in MDR Chapter II 3.1.5 in annex XV/IVDR Part A 2.3.2 (g) in annex XIII)
Statement of conformity
The following documents will be public:
Scientific opinion/Expert panel views
Description of the arrangements to comply with the applicable rules on the protection and confidentiality of personal data/personal information
The following documents will be public only once the End report and summary of the CI/PS or PMCF/PMPF are published.
Clinical investigation/Performance study plan
PMCF/PMPF investigation plan
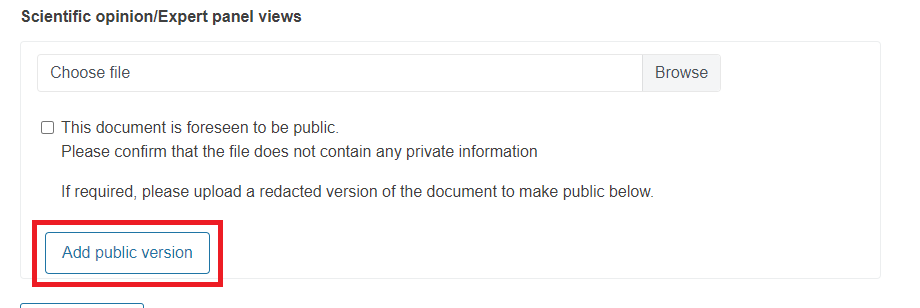
For these documents, you will be asked to confirm that they do not contain private information. To do it, you must tick the box.
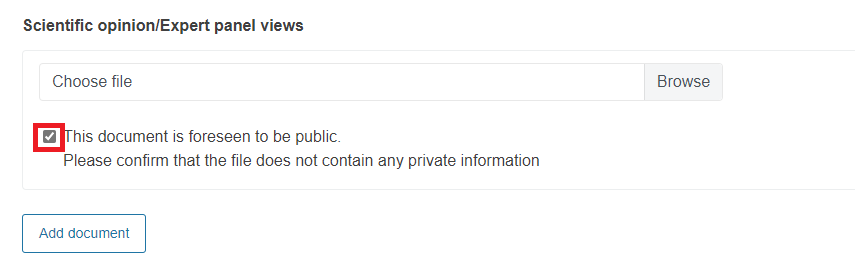
If the document has private information, do not tick the box. Instead, you must upload a redacted version of the document to make it public.
On the clinical/performance evaluation plan, reply Yes or No to the question Will the relevant document(s) be uploaded?
If you reply Yes, you will be asked to provide the Clinical/Performance evaluation plan.
If you reply No, you will be asked to provide the Clinical/Performance evaluation plan reference number.
At the end of the screen, acknowledge the Ethics Committee statement, i.e., the Competent Authority may contact the Ethics Committee that is assessing or has assessed the application.
