Economic operator information
At least one EO must be identified. Note the system message before proceeding:

If you know the Actor ID/SRN, click the box and enter at least five characters. Click Find and select the intended EO:
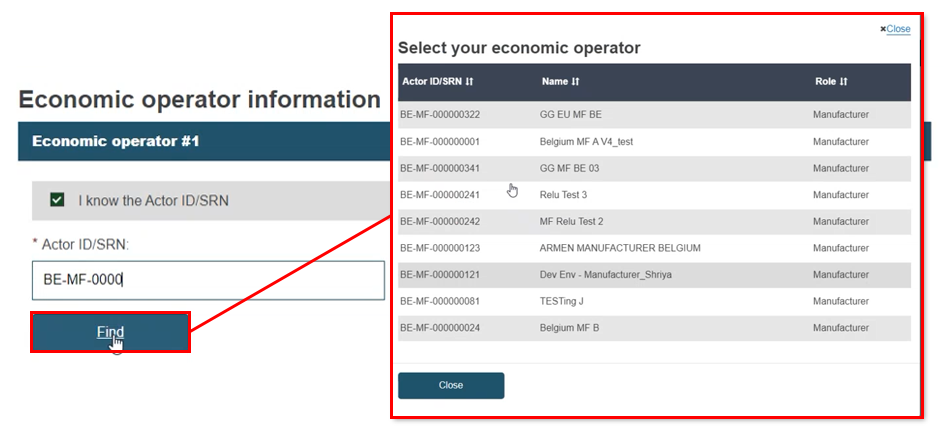 |
The system pre-populates the EO information fields, and the EO contact information can be edited. If you register new contact information, it only relates to this Market Surveillance procedure. You can change your choice of EO by clicking Change economic operator and follow the same steps:
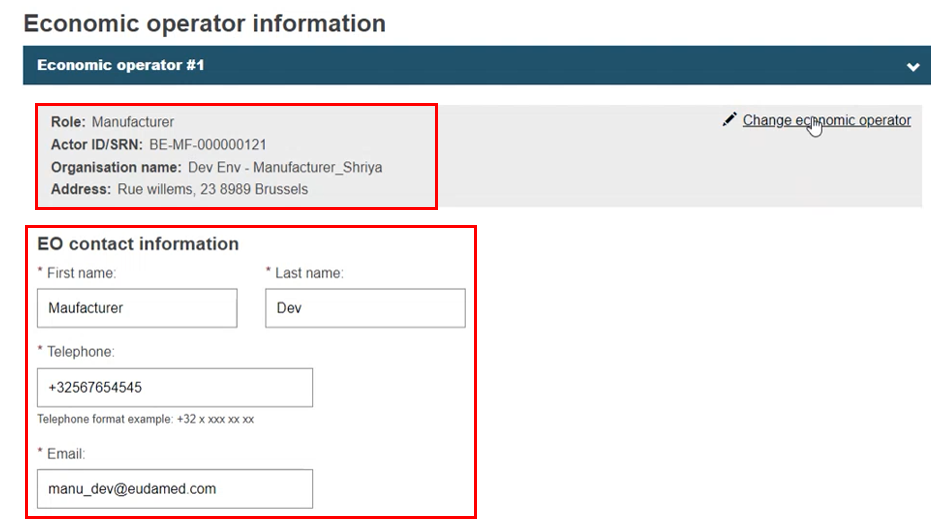 |
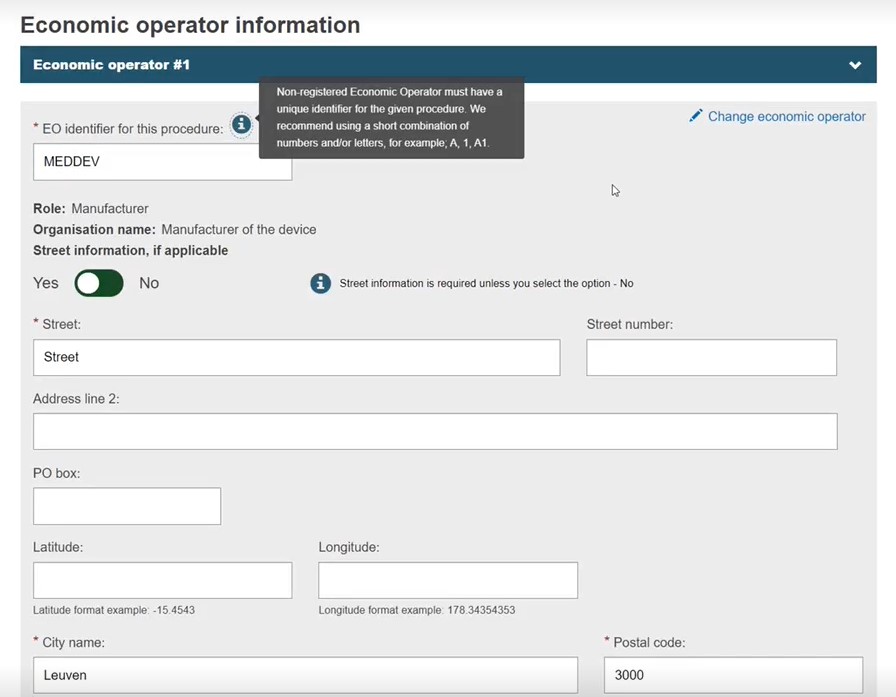
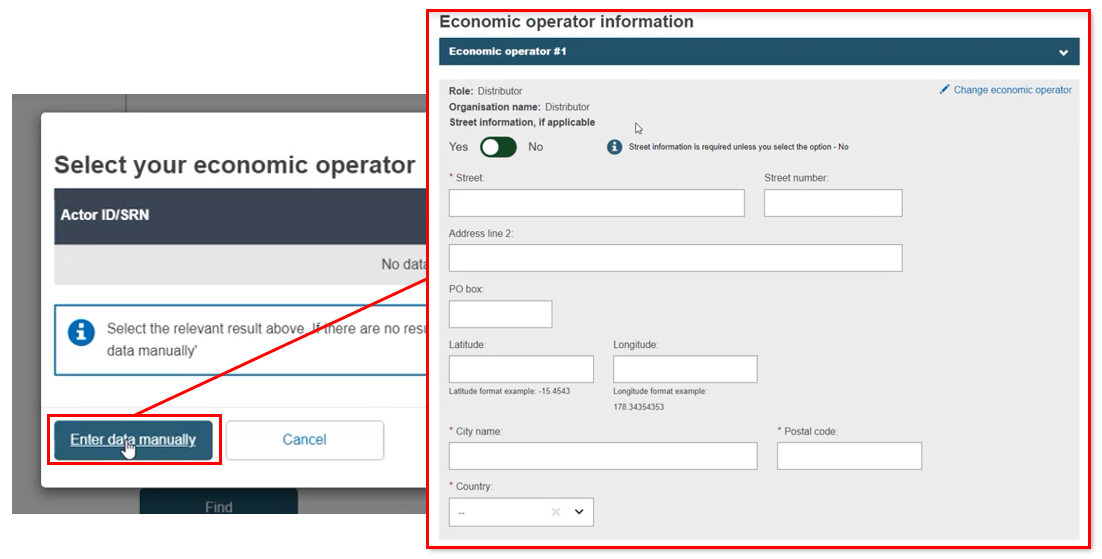
If the EO is not registered, enter the data manually.
Provide a unique EO identifier (up to 20 characters), which is used when linking a device to it.
While a Distributor is not an actor in EUDAMED, it can be linked to a procedure. Select Distributor, type in the Distributor name and click Find. As no result will be shown, click Enter data manually and complete the distributor information:
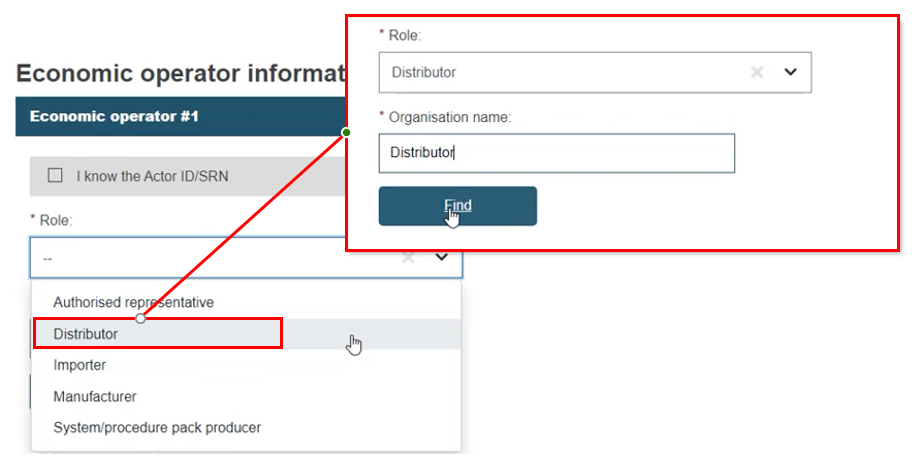
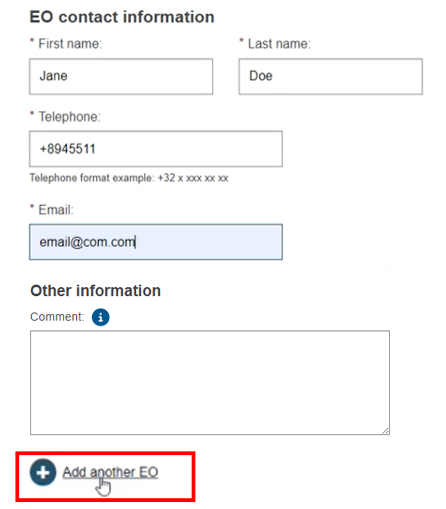
To add additional EOs, click Add another EO:
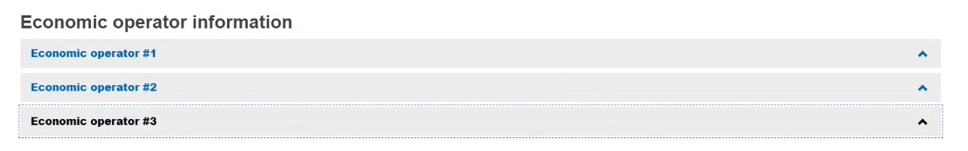
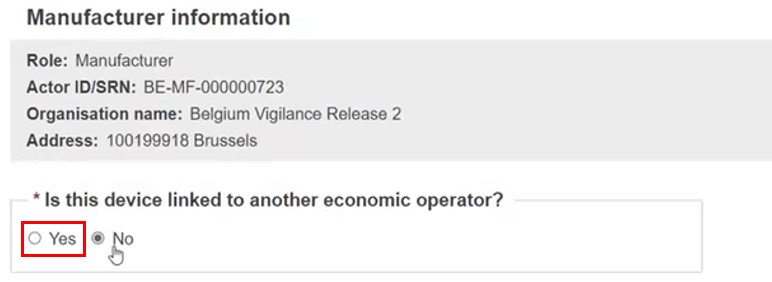
Every EO must be linked to at least one device (see Device information). This could be ensured by linking EO(s) to the device in the device section. Manufacturers and Authorised Representatives of the registered device are linked automatically.
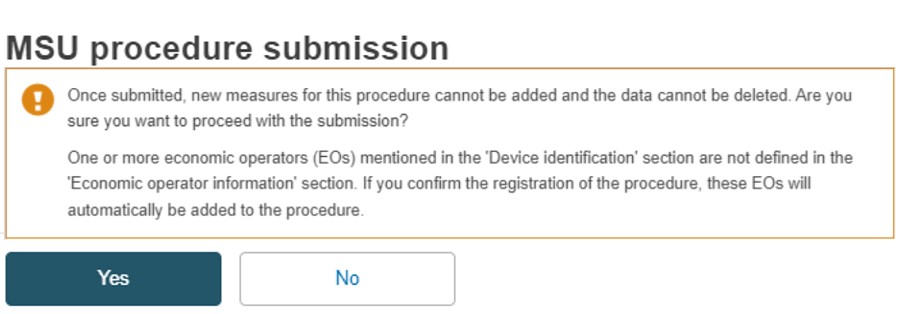
Note
On submission, if a Manufacturer or Authorised Representative referred to in the procedure Device is not linked to the Procedure, the confirmation warning displays.
EO contact information fields are automatically populated when submitted a procedure or the new version of the procedure:
 |