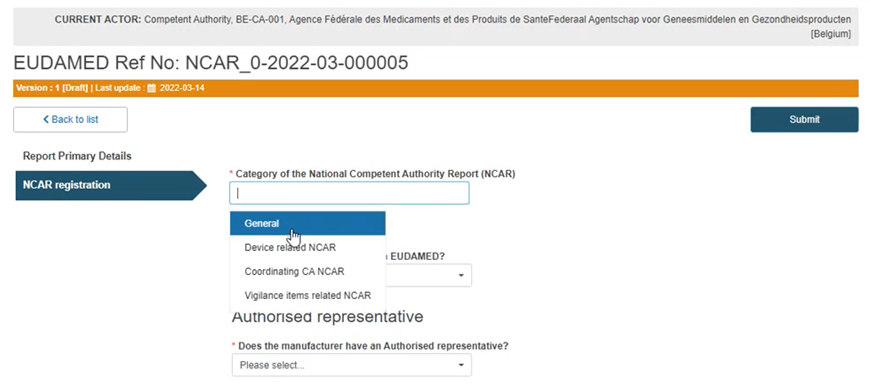
Case A: General NCAR
Select the General NCAR category in the first field:
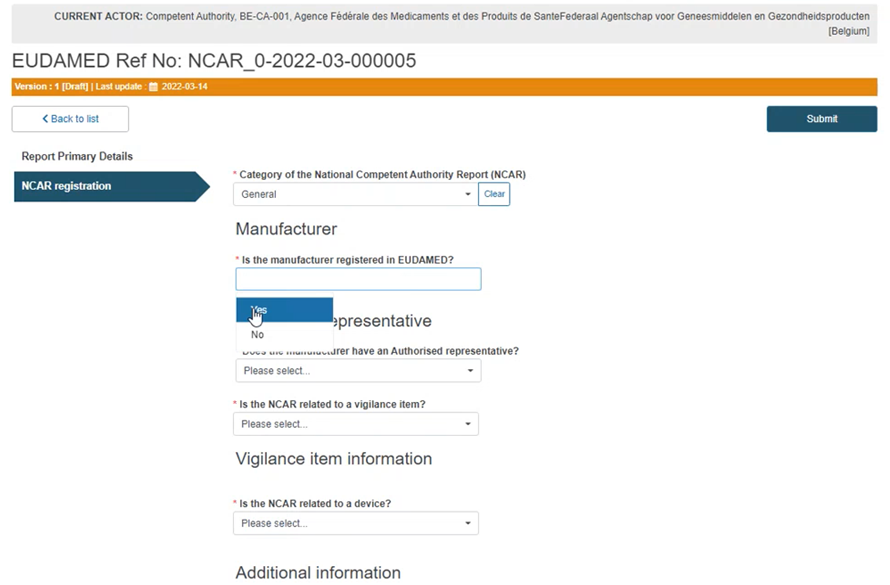
Select Yes or No in the Manufacturer field, as shown below:
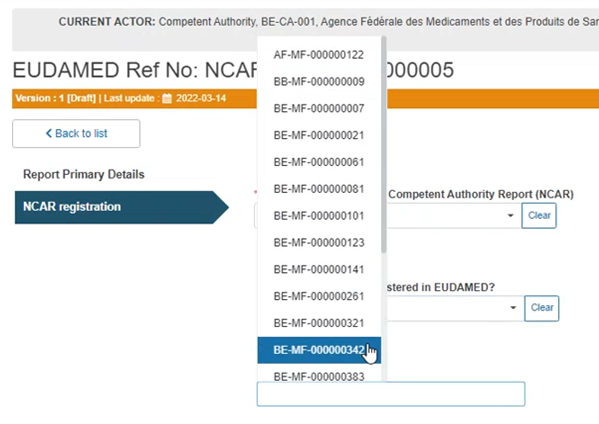
If you answered Yes to the previous question, you have to select the Actor ID/ SRN from the drop-down list in the field that will appear below:
Specify if the Manufacturer (MF) has an Authorised Representative (AR):
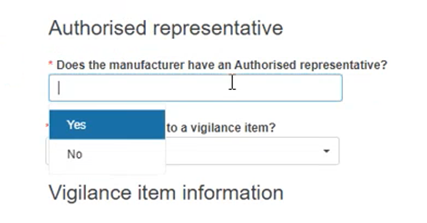
Specify if the NCAR is related to a Vigilance report:
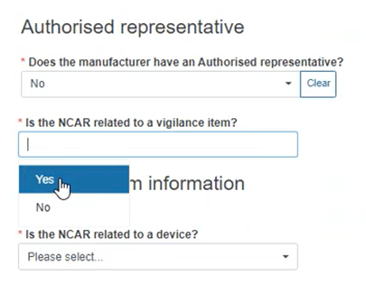
If Yes, provide the Vigilance item information below:
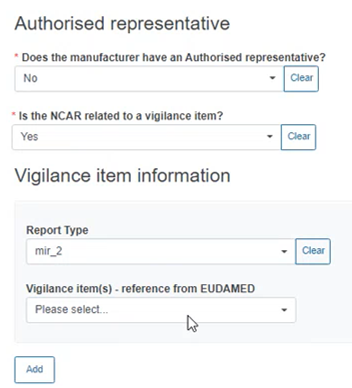
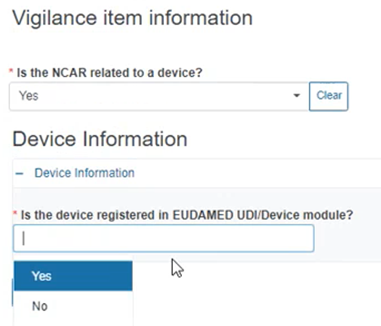
Specify if the NCAR is related to a device:
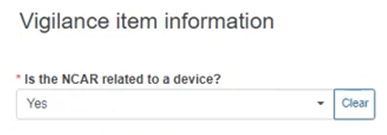
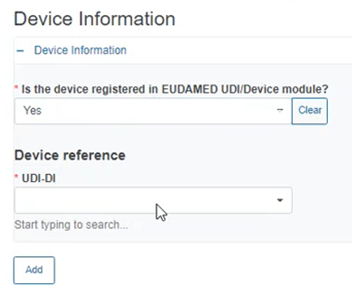
If Yes, provide the Device information below:
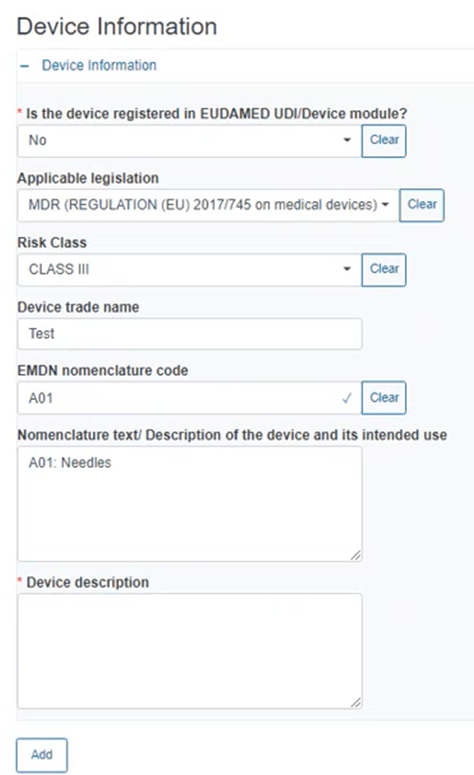
If No, fill in the information in the appearing fields including Applicable legislation, Risk Class etc., as shown below:
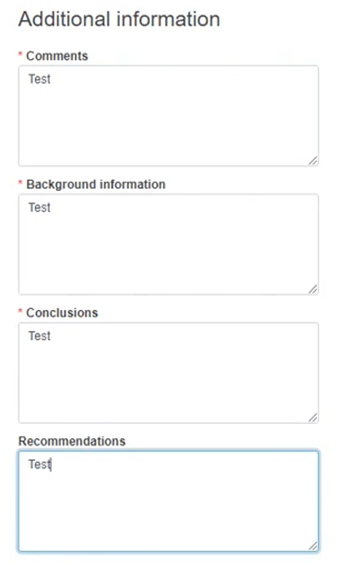
Provide any useful and relevant comments in the description fields under Additional information:
Click on Browse under Related documents to attach any documents relevant to the information provided:
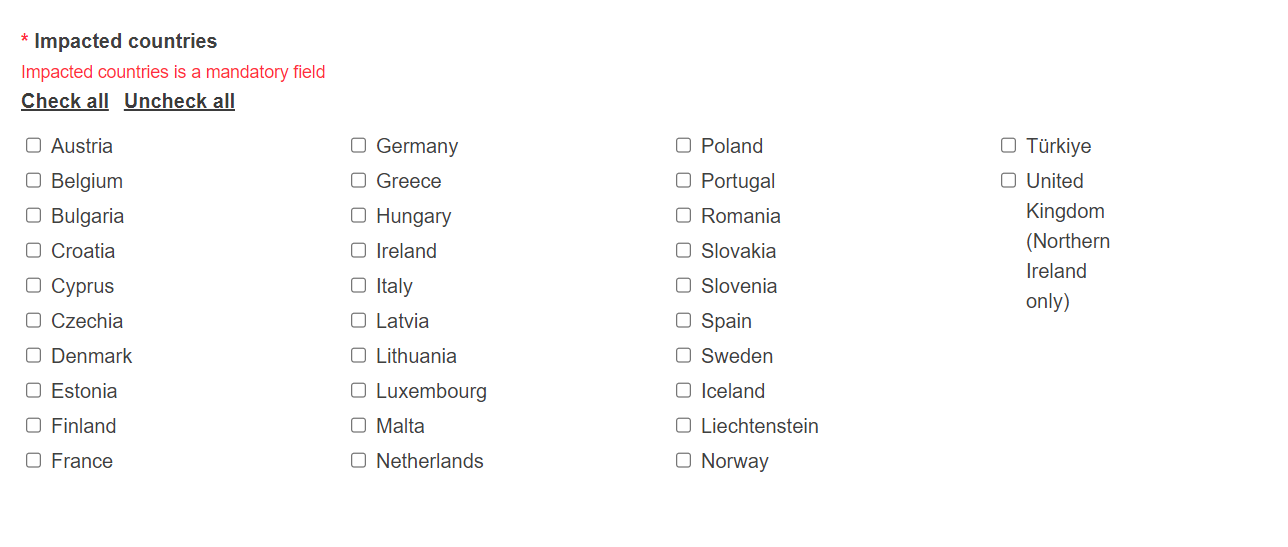
Complete this section by ticking the impacted country(/-ies), the Competent Authorities of which will be notified about this NCAR:
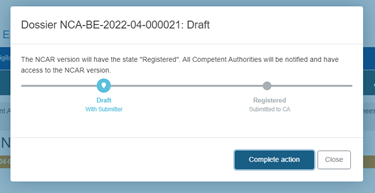
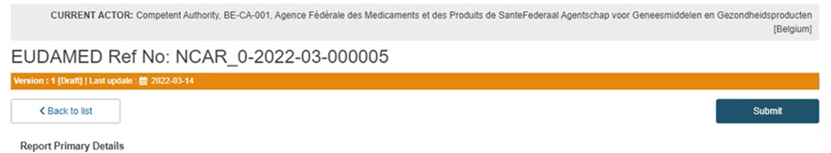
Submission:
Submit the report by clicking on the blue Submit button on the top right corner:
Finalise the submission of the report by clicking on Complete action in the pop-up window: