Authorisation process
Note
The authorisation process focuses on the technical aspects of the application.
Important
A Sponsor is not allowed to start a CI/PS application without the approval of the CA. The CA user must have a confirmer role to be able to authorise CI/PS applications.
Note
If during the validation process the CA selected Validated (may start), no further action is needed for the concerned application.
Once the application is authorised or refused, the CA cannot perform any further action.
Note
The CI/PS application will become available on EUDAMED public portal when it is authorised.
Once the application has been validated but authorisation is needed, the CA can perform several actions to move the application forward.
Note
The view below applies to CI/PS application – one country (first submission).
Under the tab Report history overview you can follow the authorisation progress of the application.
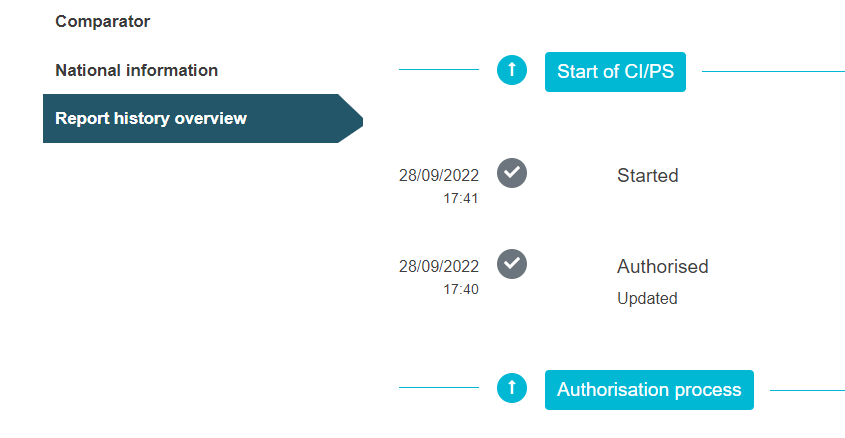
For more information about the indicative deadlines of the authorisation process, see chapter Deadlines applying to the CI/PS application – one country.