New version and devices
Each device scope type allows different levels of edit or update. These are shown in the tables below, and they apply to all procedure types:
Possible device scope type actions:
Device scope type description | Registered / Non-registered | Device may be changed | Device may be removed | Device information may be edited |
|---|---|---|---|---|
Name (No UDI / EUDAMED DI) | Non-registered | No | Yes | yes |
UDI-DI / EUDAMED ID | Registered | No | No | No |
Non-registered | No | Yes | Yes | |
Basic UDI / EUDAMED DI | Registered | No | No | No |
Non-registered | No | Yes | Yes | |
Category | No | Yes | Yes (EMDNs may be added or removed) | |
Production identifier (UDI-PI) | Production identifier data | No | No | Yes |
Possible actions for a device linked to Production identifier data:
Registered / Non-registered | Linked to PI Device may be changed | Linked to PI Device may be removed | Linked to PI Device information may be edited |
|---|---|---|---|
Registered UDI-DI / EUDAMED ID | No | No | No |
Non-registered and device identifier is not available (not known) | No | No | Yes |
Non-registered and device identifier is available (known) | Yes (using 'Check registry' functionality) | No | Yes |
Example scenarios:
The Device scope type is always greyed out, not editable and cannot be removed. In this case we use the minimal Name identifier that can only link to non-registered devices, for which the fields can be edited and EO(s) must be identified.
If however, information for the UDI-DI/EUDAMED DI or other device scope types become known and you wish to link the device to the procedure using these instead, click Remove category/link/device and add a new device based on the new information (see Section Device information).
For a registered device, Remove category/link/device function would not appear:
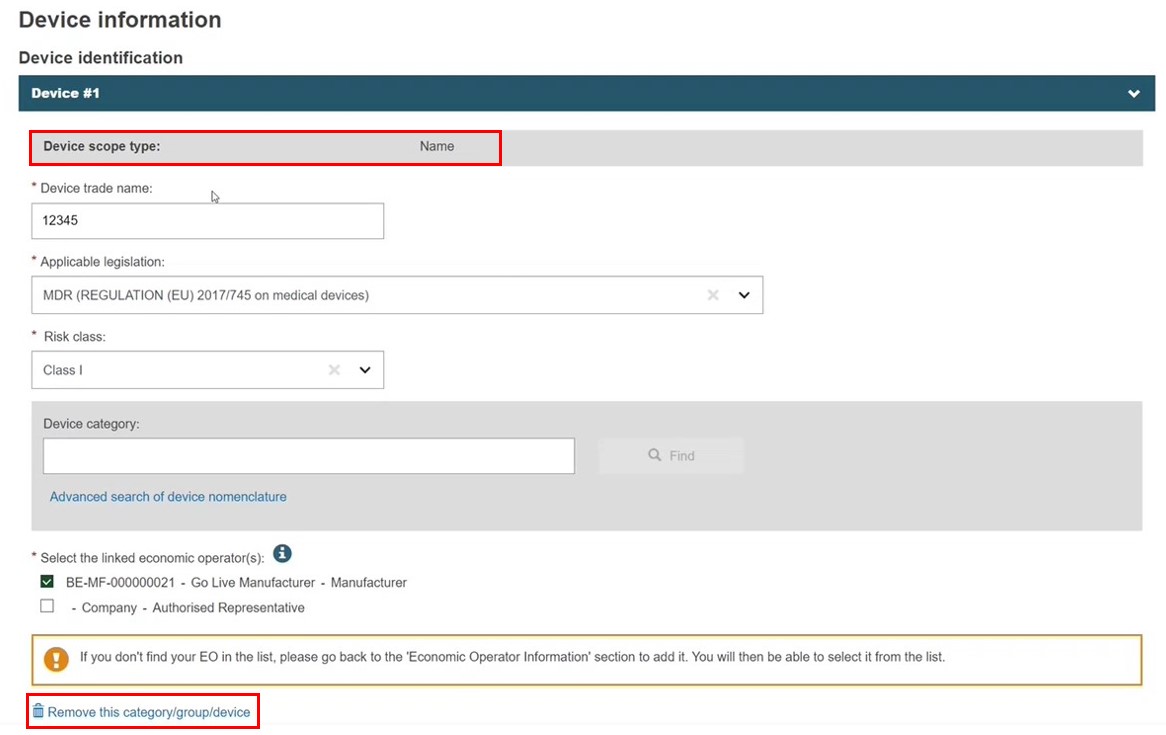
Here the device is registered to the procedure with a UDI-PI, so it cannot be removed. The Device scope type is fixed, but the PI type and values are editable. The UDI applicable option cannot be changed to No:
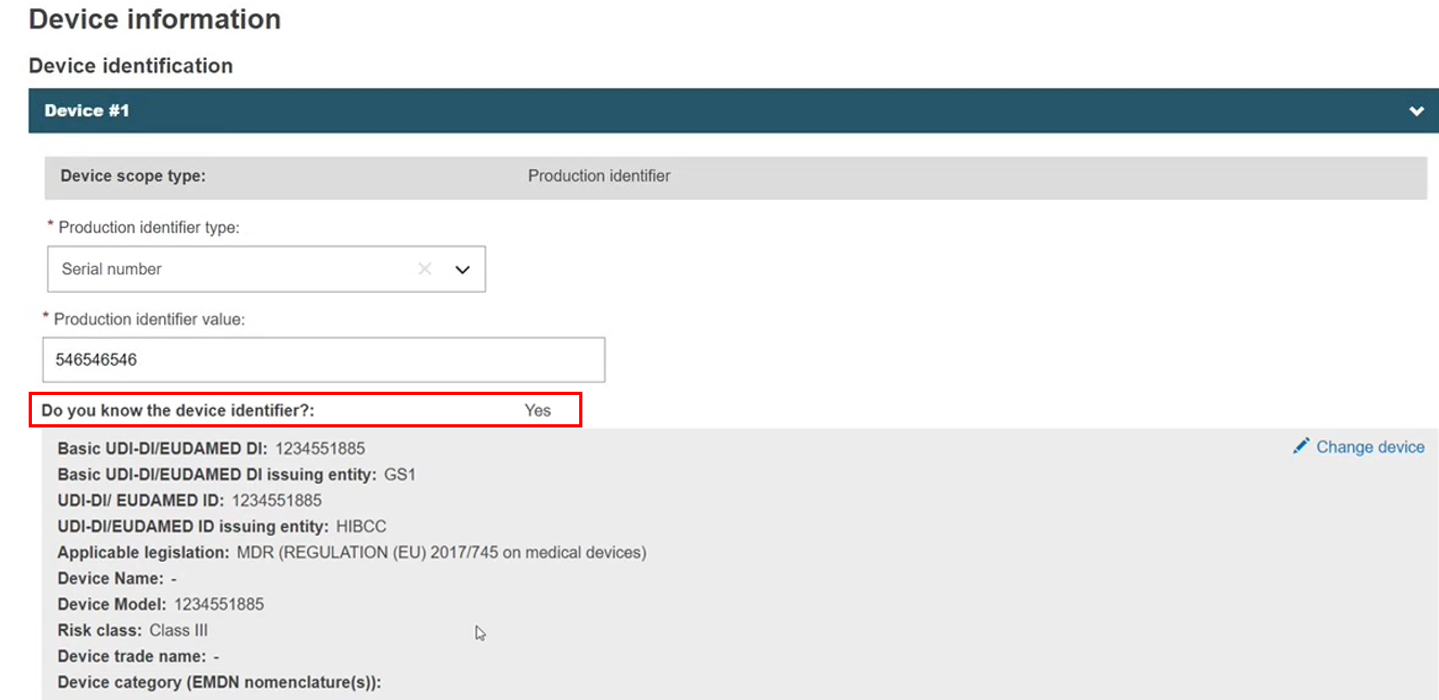
However, a non-registered device linked to the procedure with a UDI-PI device scope type can be removed, and the Do you know the device identifier? question would allow a No to Yes change.
If, in the meanwhile, the device has been registered in EUDAMED, you can link to this registered device instead. Here we see data provided through the device identifier only, so create a new procedure version to allow this to be updated.
Provide the UDI-DI/EUDAMED ID and click Check registry. If system finds the exact match, you can select the device:
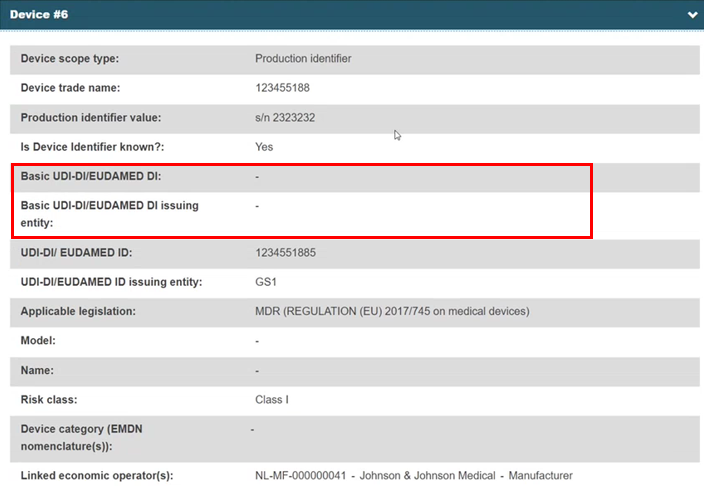
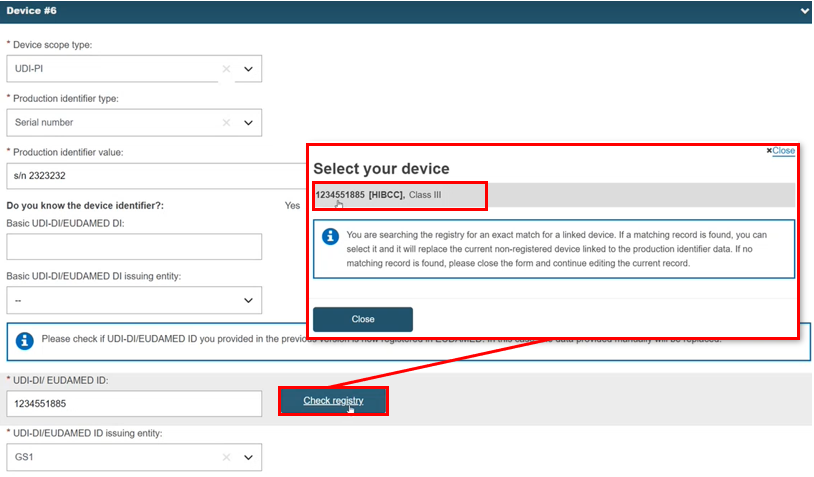
The system replaces the non-registered device with the registered device and retrieves the data:
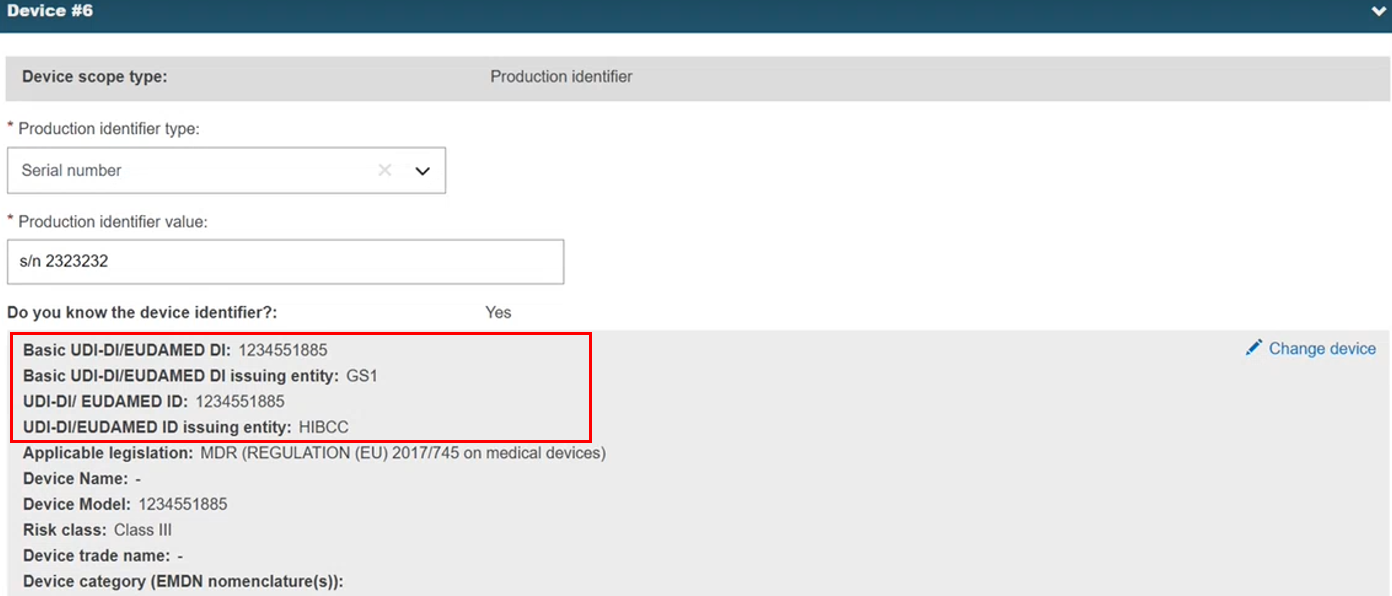
Click Submit new version. The registered device is linked to the procedure. If the device is not found, the user can close search result pop-up window and continue editing the procedure.
Note
In the new version, when replacing a non-registered device with a registered one for the Production Identifier scope type, the linked EO(s) remain linked. The user can now update the EO link(s) if necessary.