Managing mandates
Specific to non-EU manufacturers and authorised representatives
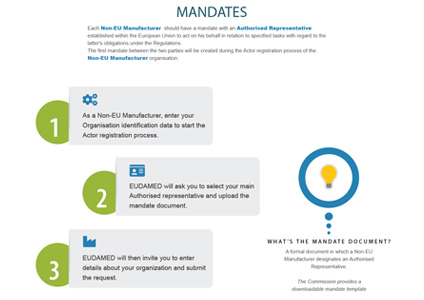
When registering in EUDAMED, non-EU manufacturers are required to provide information on their mandate with an authorised representative.
If they have mandate(s) with other authorised representative(s), these must also be individually registered in EUDAMED, those mandates need to be verified by the concerned authorised representative.
Additionally, the Authorised Representative or the non-EU manufacturer can decide to terminate the mandate at any moment.
 INFOGRAPHIC: Mandates for AR/Non-EU Non-EU MF
INFOGRAPHIC: Mandates for AR/Non-EU Non-EU MF