Case D: Vigilance items-related NCAR
Select the Vigilance items related NCAR category in the first field of the NCAR Registration section:
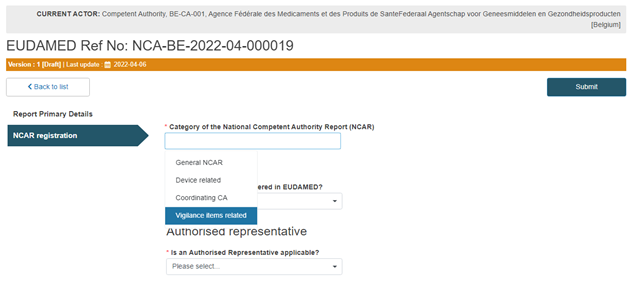
Select Yes or No in the Manufacturer field, as shown below:
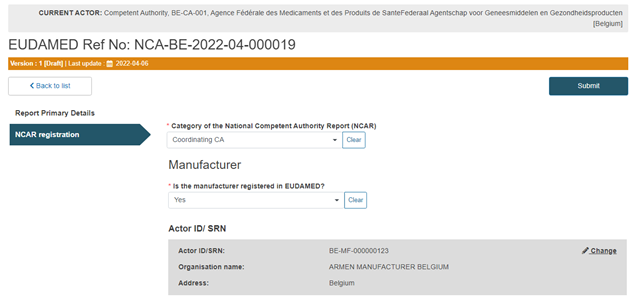
Specify if the Manufacturer has an Authorised Representative:

Provide reference to a Vigilance report:
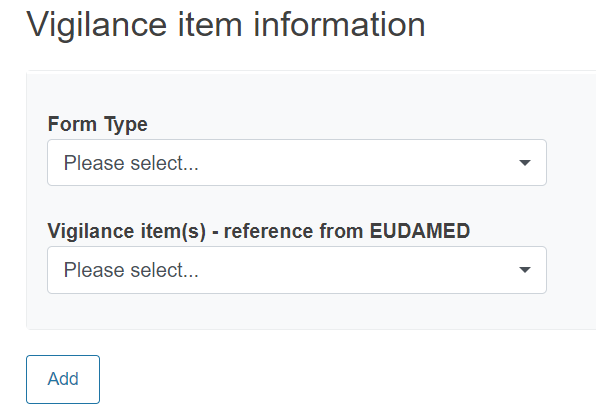
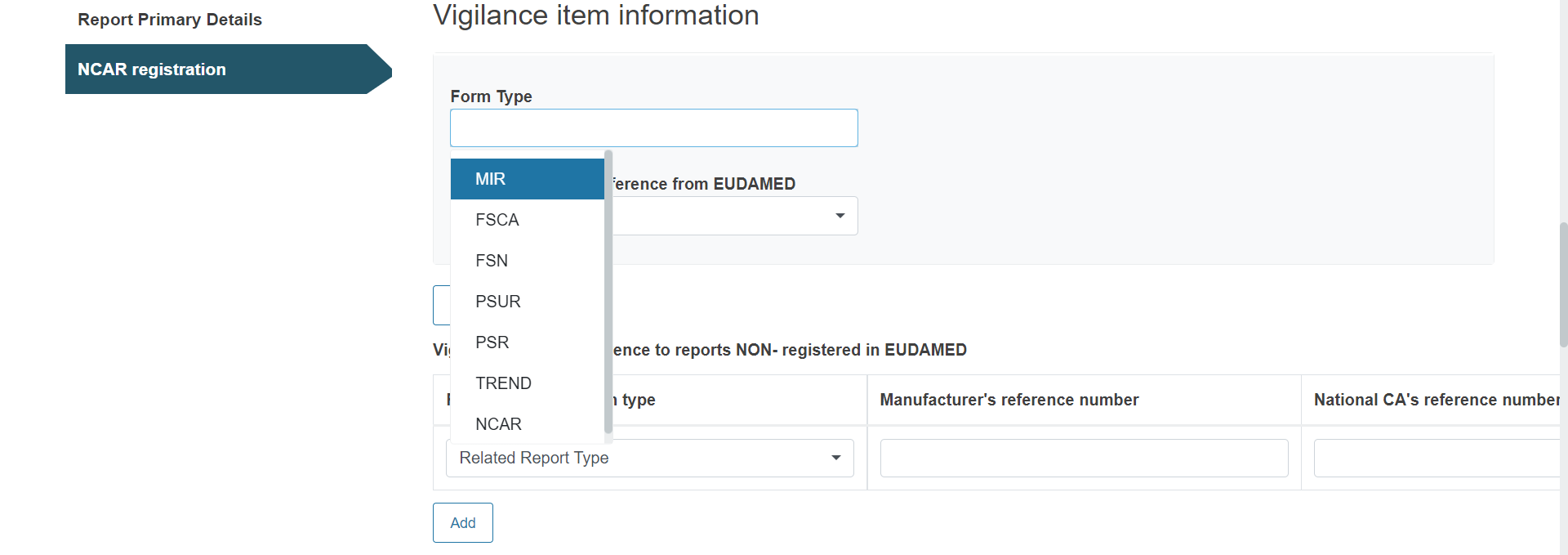
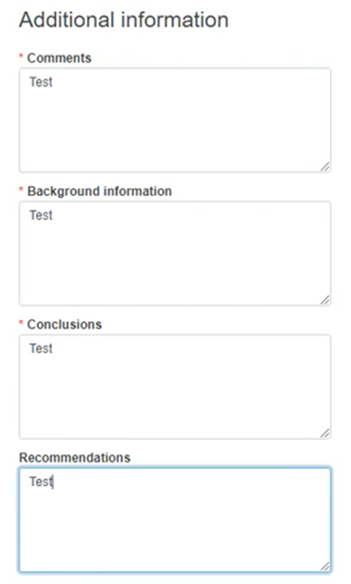
Provide any useful and relevant comments in the description fields under Additional information:
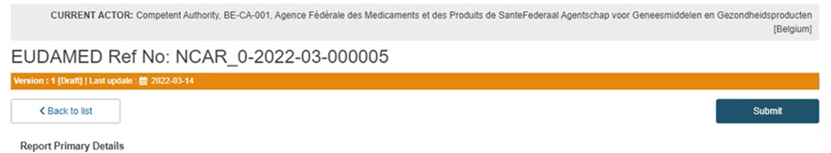
Submission:
Submit the report by clicking on the blue Submit button on the top right corner:
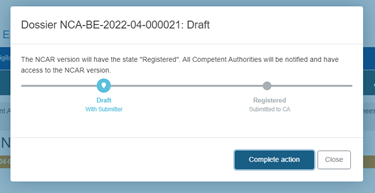
Finalise the submission of the report by clicking on Complete action in the pop-up window:
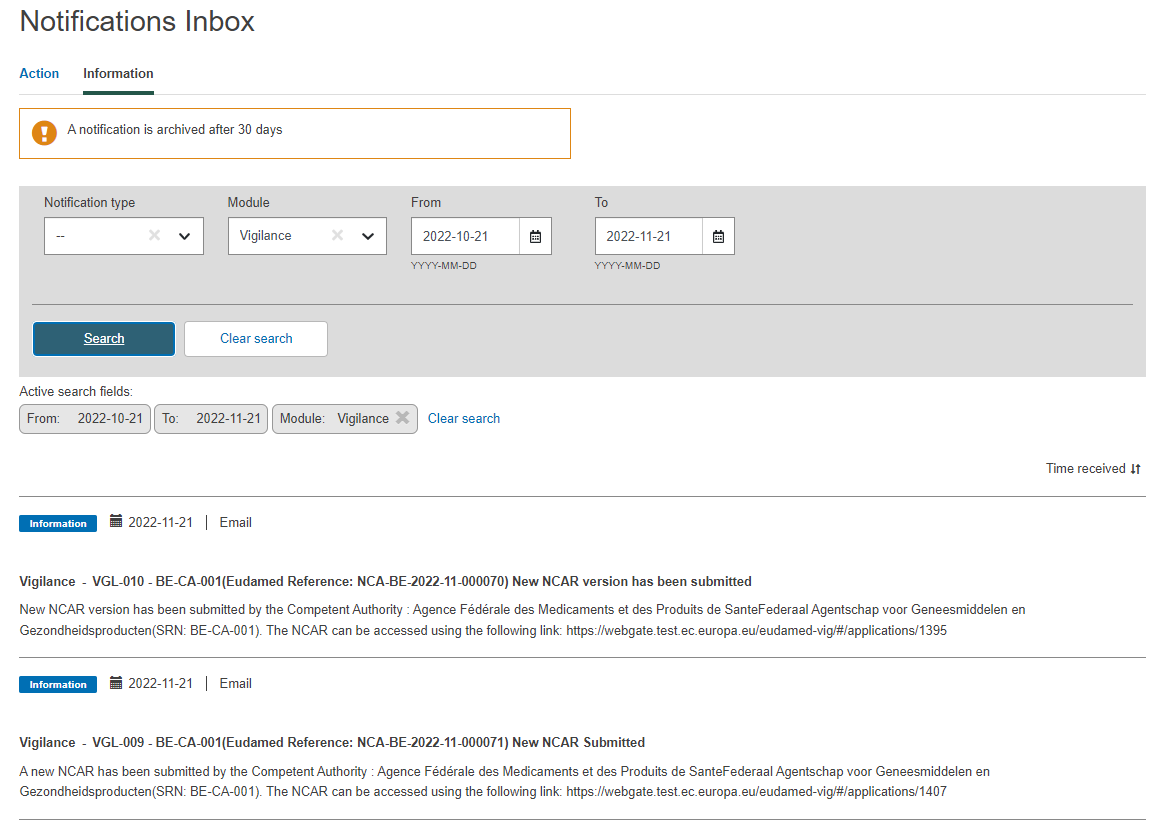
Note
CAs with LAA profile only that were selected in the report, after submitting or updating the version of a NCAR form, will receive a notification in their Notifications inbox (Information tab):
 |