Incident information
Click on the Incident information section from the menu on the left:
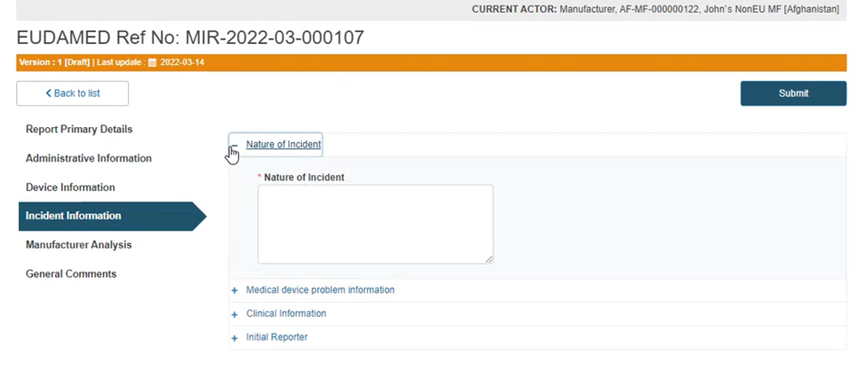
Click on the plus sign next to the first segment, Nature of incident, and add a description in the text box:
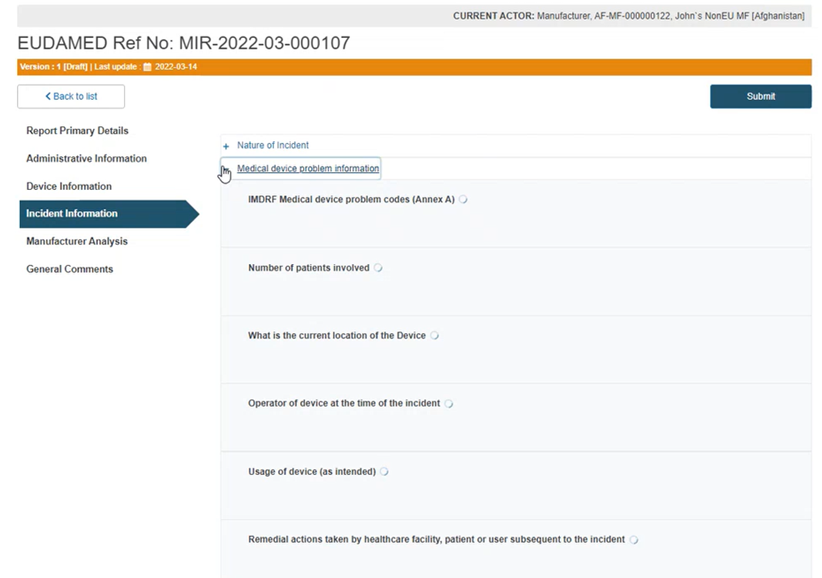
Similarly, click on the plus sign next to Medical device problem information and complete the requested information:
Select Yes to specify IMDRF code(s) to define the device problem:
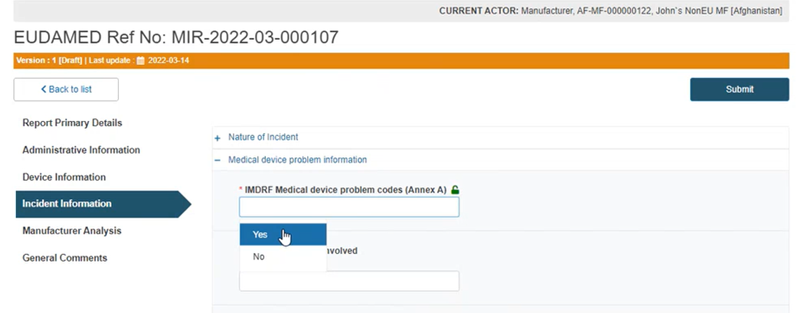
Type and choose a code or title in the box below:
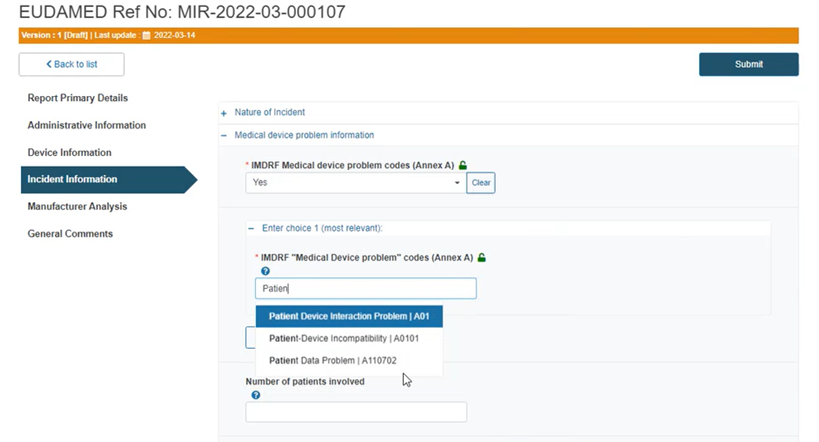
If you select No, reply to the question If you think the incident is unique and a suitable IMDRF term is missing, briefly explain.
Tip
Find the correct IMDRF codes
Click on the blue question mark above for a link to the entire list of IMDRF codes via a pop-up window:
The link will redirect you to the IMDRF Codes list for the specific Annex.
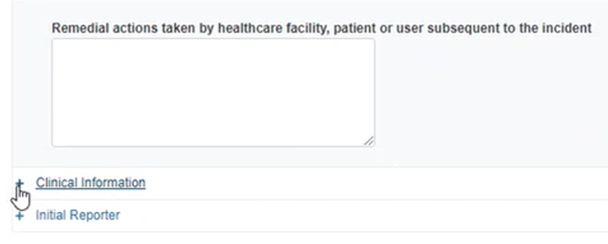
Insert the number of patients involved, select the current location of the device, the operator at the time of the incident, the usage of the device and a description of remedial actions:
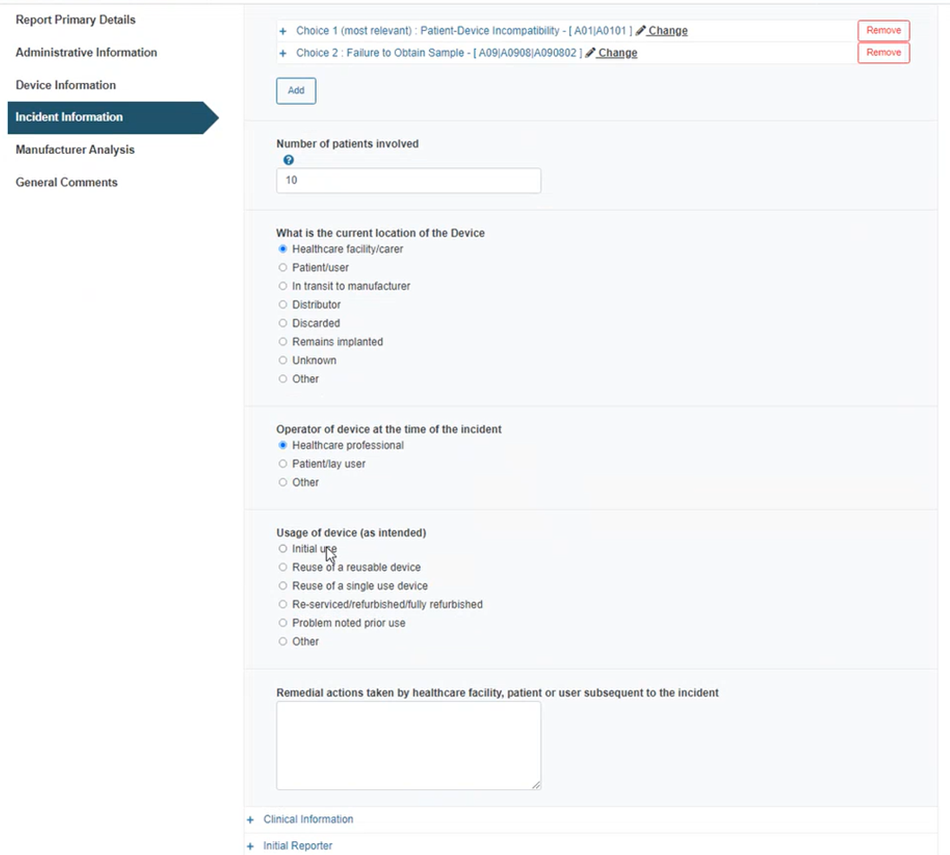
Click on the plus sign next to Clinical information:
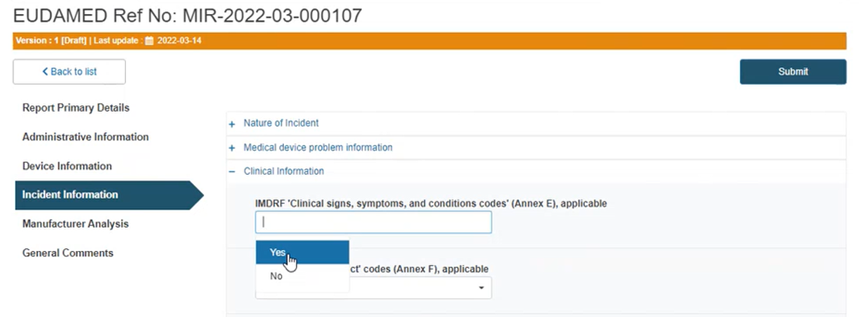
Select Yes or No if the IMDRF codes (Annex E and Annex F) are applicable. If No provide an explanation in the text box below:
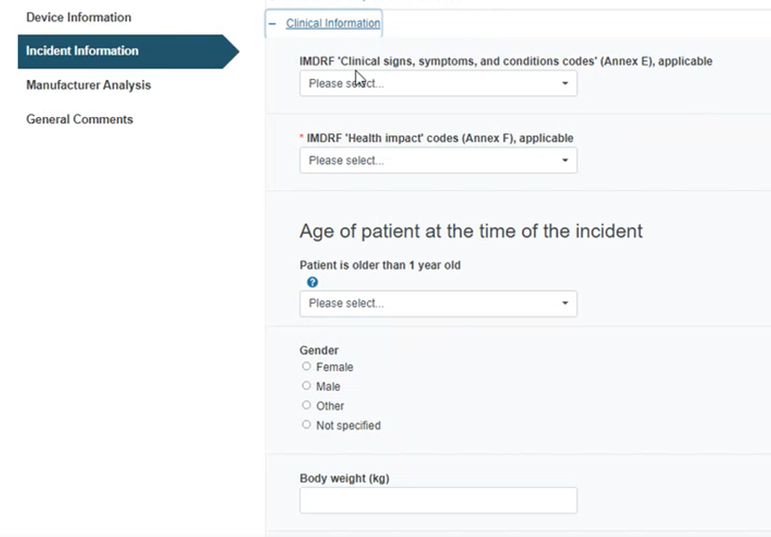
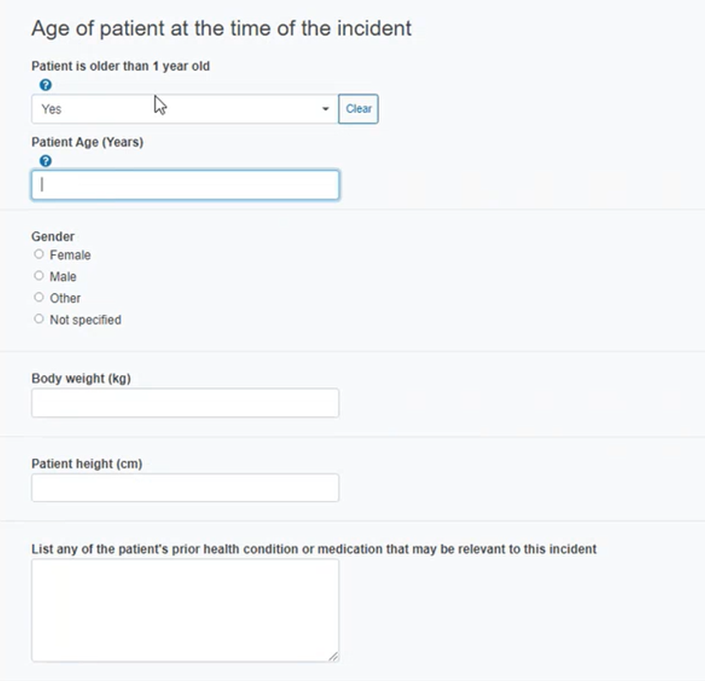
Provide the patient’s age and other details in the section below:
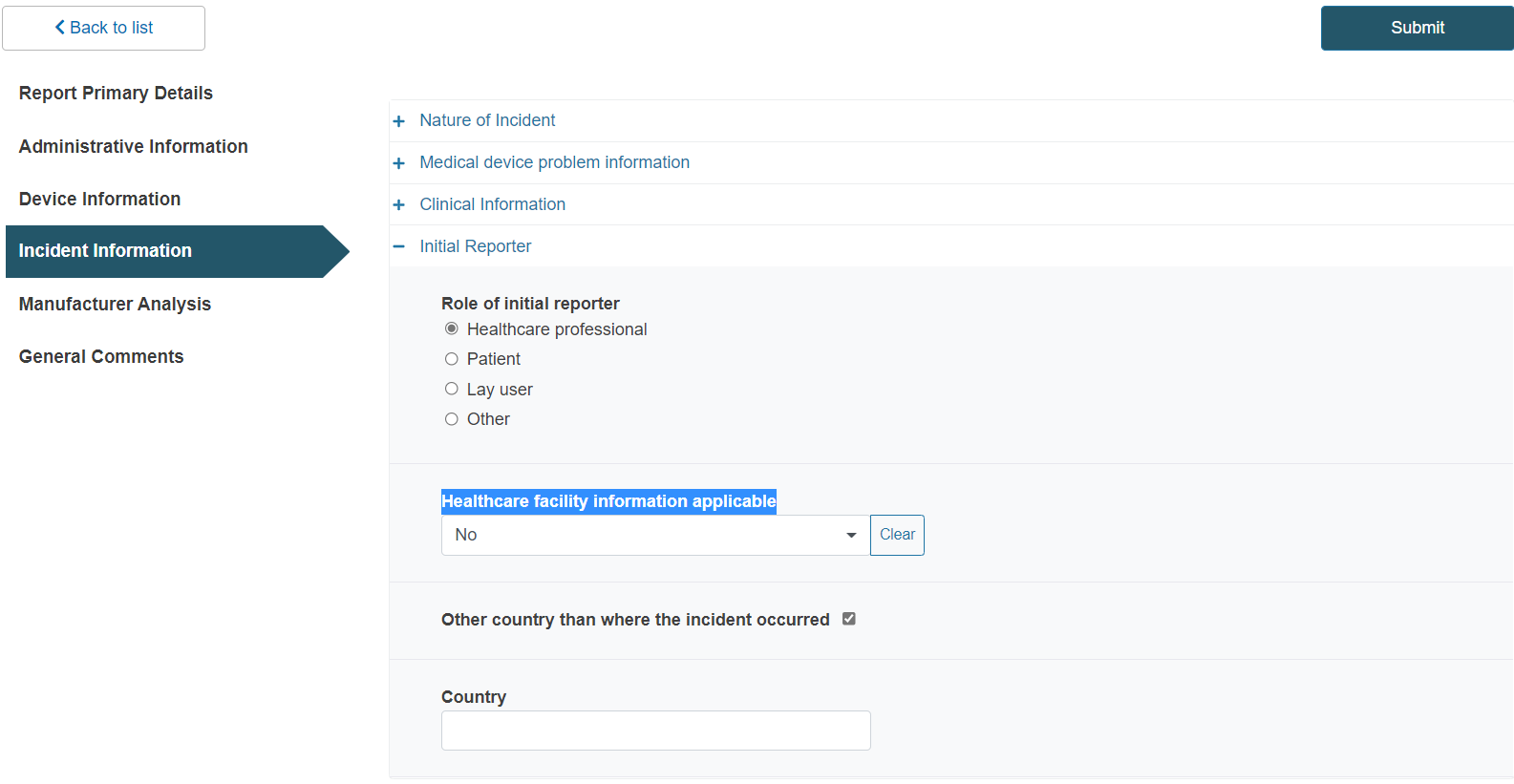
Click on the plus sign next to Initial Reporter:
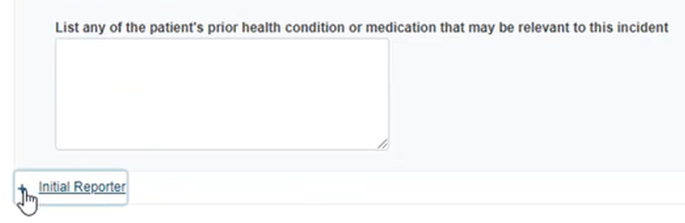
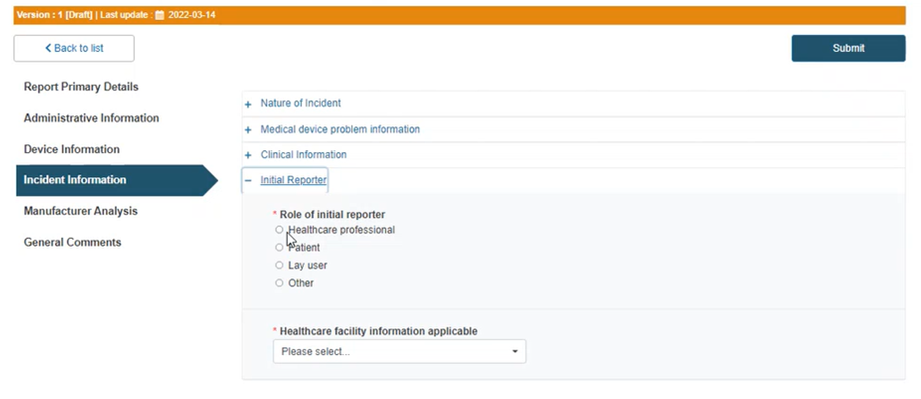
Select the initial reporter:
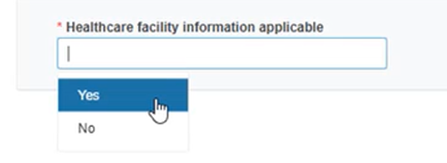
Select Yes to provide relevant healthcare facility information if applicable and fill in the details:
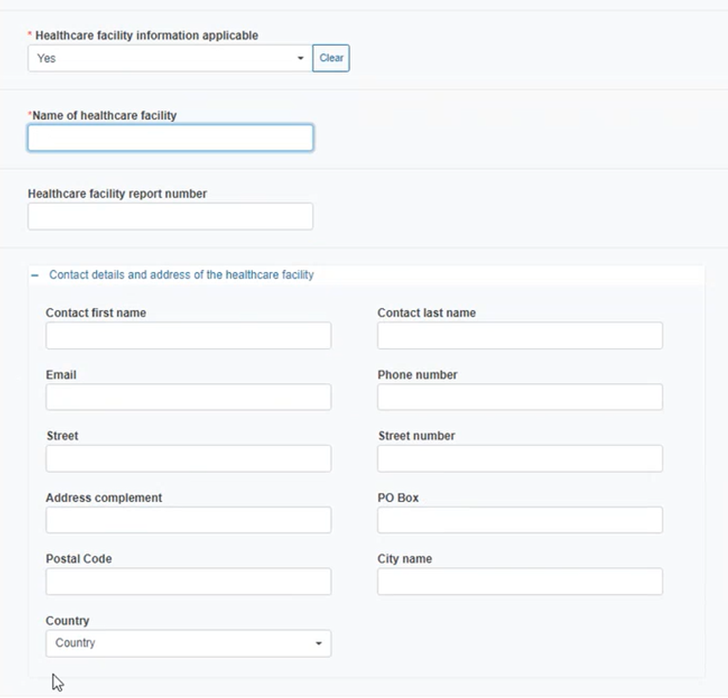
If you answered No, tick the country box and type the healthcare facility country in the emerging field in case the country is different to the one where the incident occurred: