Case A: Device with a known UDI-DI/ EUDAMED ID
If the device UDI-DI (or EUDAMED ID in the case of legacy devices) is known, answer Yes to the question Does the device have a UDI-DI/ EUDAMED ID that is known?:
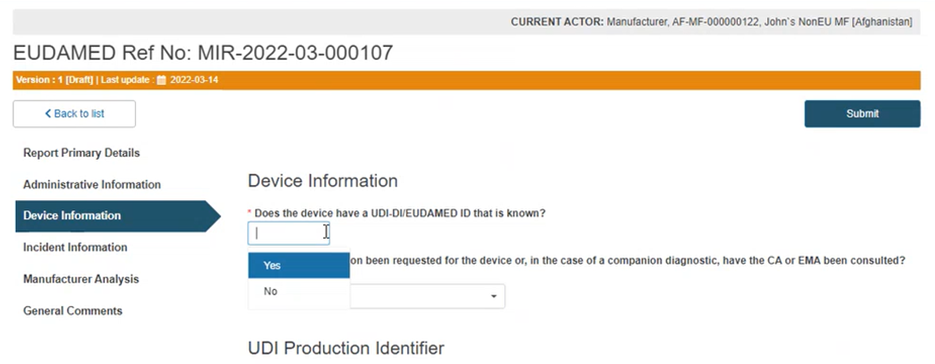
Answer Yes or No to the question Is the device registered in EUDAMED UDI/Device module?:
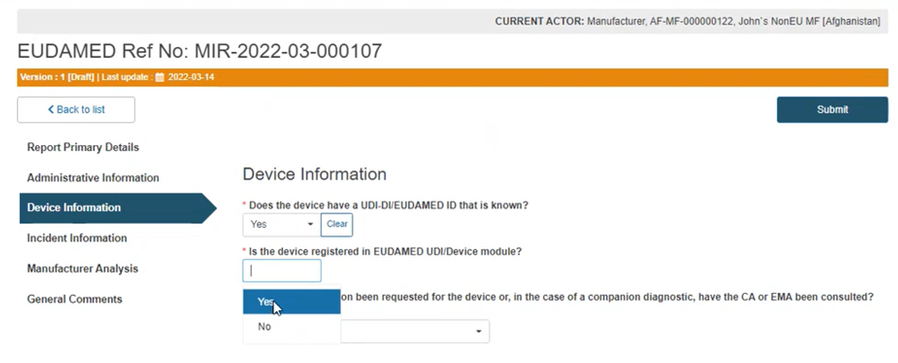
If you answered Yes, start typing the UDI-DI/EUDAMED ID of the device and select the correct one:
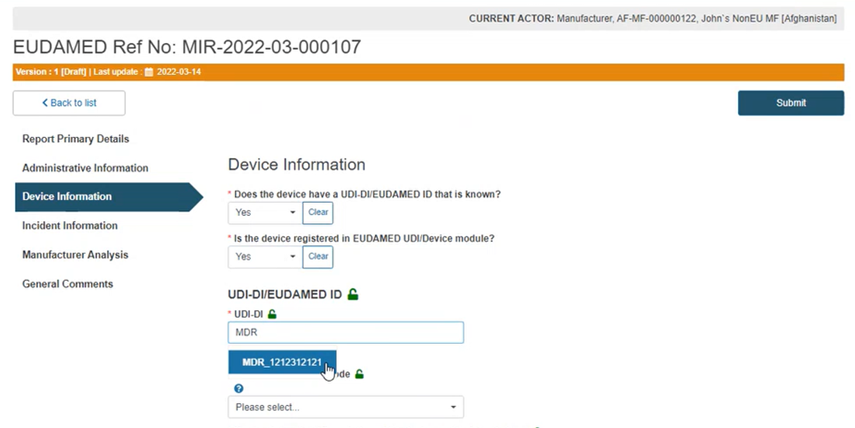
The system will retrieve all the properties of the device:
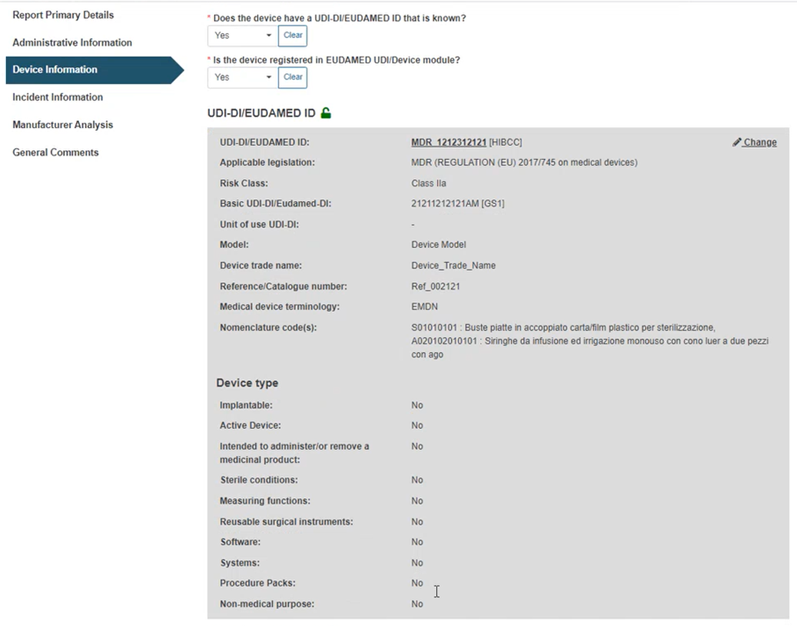
Specify the device nomenclature from the dropdown list of EMDN codes that have been already assigned to the specific device (the system will auto-fill the Nomenclature text field below):
Describe the device and its intended purpose:

Answer if a scientific opinion has been requested or – for a companion diagnostic – if the Competent Authority or EMA have been consulted:
