Basic UDI-DI identification information
 VIDEO: UDI and medical software devices
VIDEO: UDI and medical software devices
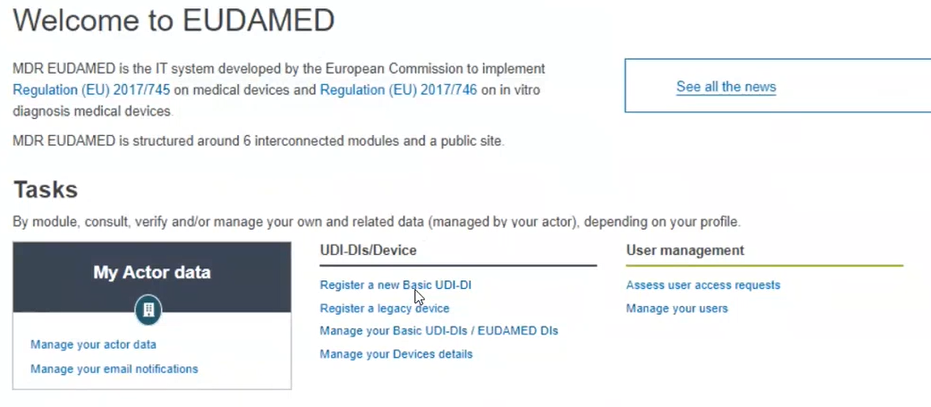
Click on Register a new Basic UDI-DI:
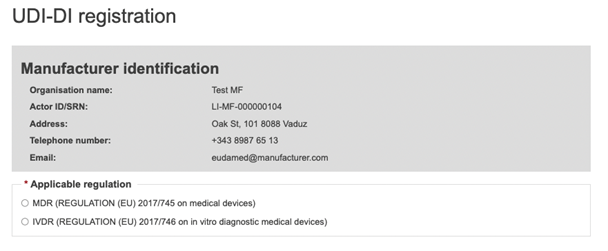
Next, enter the Basic UDI-DI information. Select the applicable regulation.
Note
In this guide, the selection is MDR (Regulation (EU) 2017/745). Based on the regulation you choose, the characteristics of the Device to be entered will vary.
Depending on the regulation selected an additional question appears at the bottom of the page:
Regulation
Additional question
MDR
Is it a System or Procedure Pack which is a Device in itself?
+ additional sub-questions about the device type, depending on whether your answer is Yes or No to this first question
IVDR
Is it a kit?
+ additional sub-question about the device type, if you answer No to this first question

If you select No, please choose the right information under the appearing section Special Device type (for IVDR, if you select No for Is it a Kit?, the only option for Special device type if applicable is Software[12] (See video on the top of the page):

Note
If one of the following Special Device types is selected, the Master UDI-DI applies:
Standard soft contact lenses
Standard Rigid Gas Permeable (RGP) contact lenses
Made to order soft contact lenses
Made to order Rigid Gas Permeable (RGP) contact lenses.
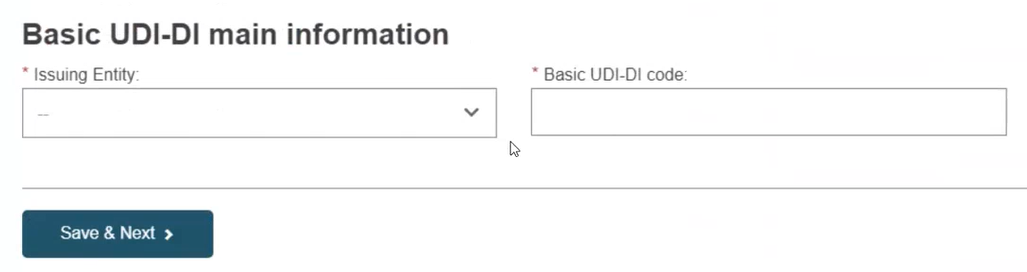
Fill in the Basic UDI-DI identification details and click on Save & Next:
Important
EUDAMED will validate the Basic UDI-DI code based on the specific format for each Issuing Entity and will prevent you from going further if the code is not valid.
If the Basic UDI-DI code already exists in EUDAMED, the system will prevent you from saving, as a Basic UDI-DI must be unique.
Non-EU manufacturers will have to select the Authorised Representative for the Basic UDI-DI amongst those with which they have an active mandate registered in EUDAMED.
If there is only one Authorised Representative with an active mandate with the non-EU Manufacturer, it will be automatically retrieved:

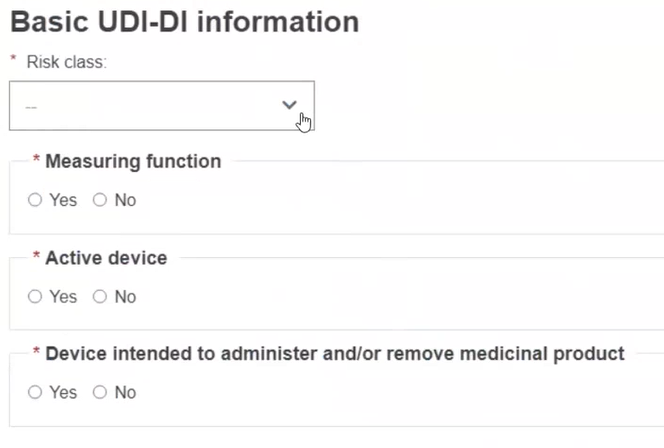
Choose a Risk Class and select Yes or No for each option that follows.
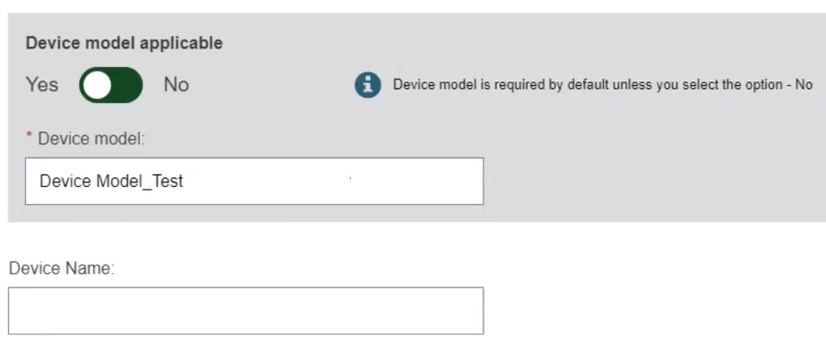
Select Yes or No if Device model is applicable. If you selected No, the Device Name will be mandatory, otherwise, it is mandatory to enter the Device model and the Device name (at the Basic UDI-DI level) if there is one (note that the device trade name is part of the UDI-DI data):
Click on Save to save your registration as a draft and continue later, or on Save & Next to save it as a draft and continue directly with the following steps:

[12] For more information, visit the EUDAMED Information Centre, or the UDI Assignment to Medical Device Software webpage.