Basic UDI-DI information
Enter the Basic UDI-DI information:
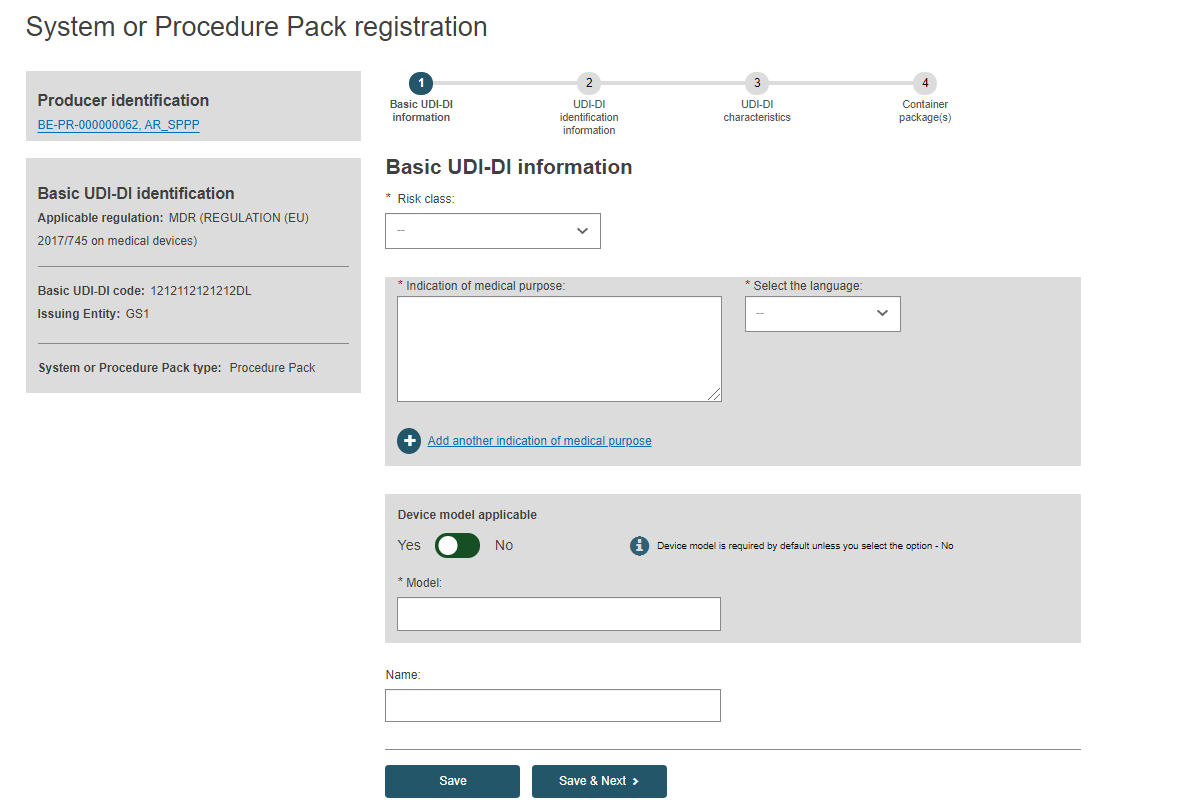 |
Choose a Risk Class from the drop-down (it must be the highest risk class of devices that are part of the system or procedure pack):
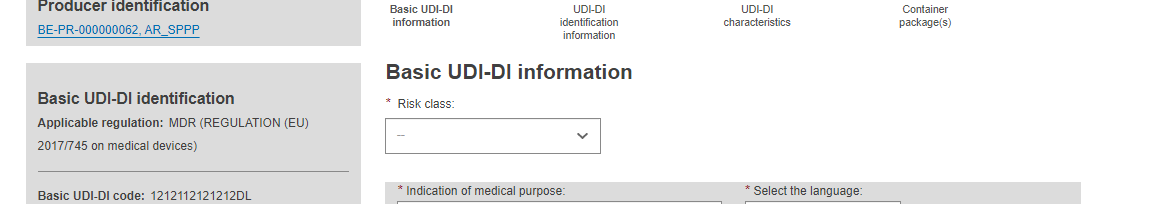
Fill in the indication of medical purpose and select the related language from the drop-down list.
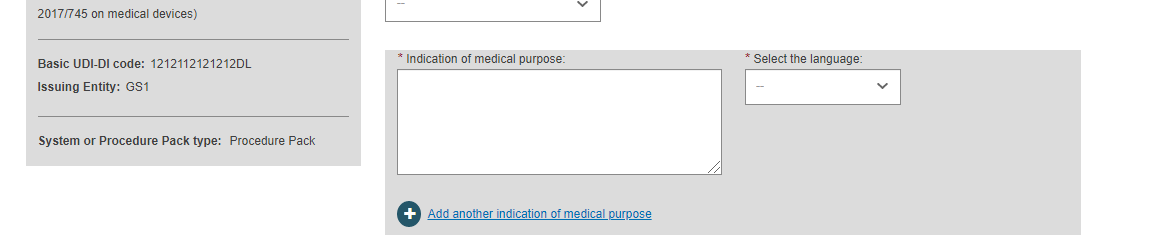
If you add the indication in multiple languages, click on Add another indication of medical purpose and select its language.
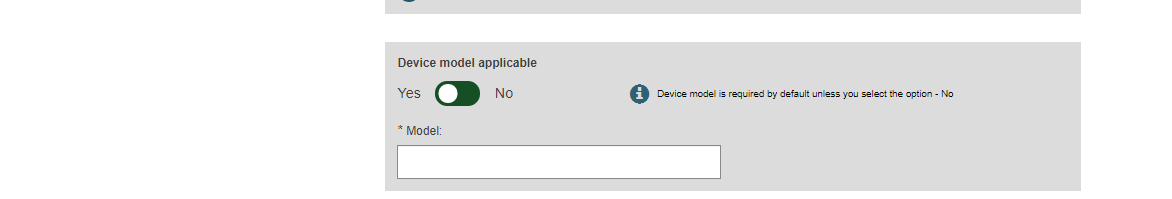
Select Yes or No if Device model is applicable and, if so, enter the Device model and a device name if there is one. Otherwise, enter only a Device name:
Click on Save to save your registration as a draft, or click on Save & Next to save it as a draft and continue to the next steps: