Manufacturer
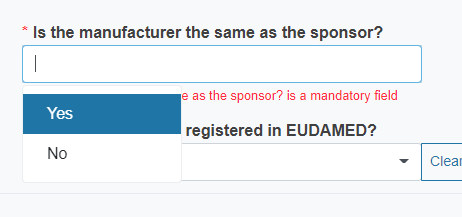
Reply Yes or No to the question Is the manufacturer the same as the sponsor?
If the device:
is not CE marked or
is CE marked but it is not registered in EUDAMED,
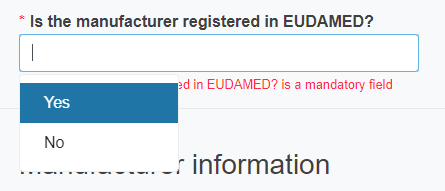
you need to reply reply Yes or No to the question Is the manufacturer registered in EUDAMED?
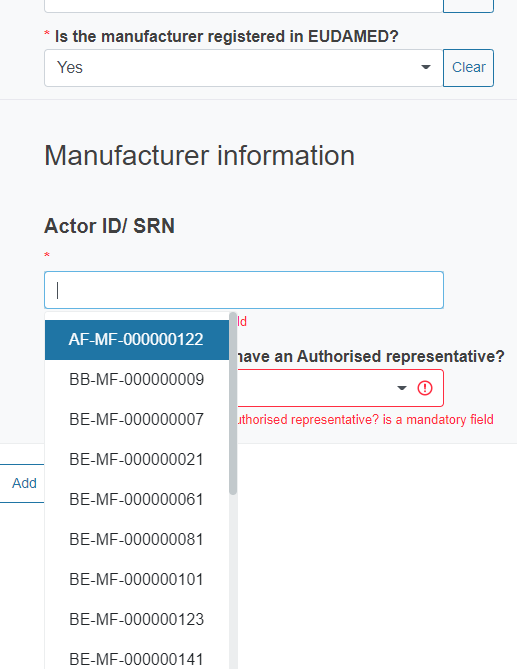
If you reply Yes, you will be asked to select from the dropdown list the Actor ID/SRN of the manufacturer.
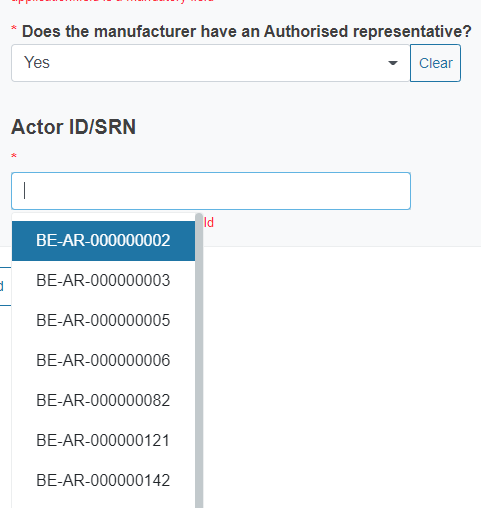
You will also have to indicate if the manufacturer has an authorised representative. If you reply Yes, you must select from the dropdown list its Actor ID/SRN as well.
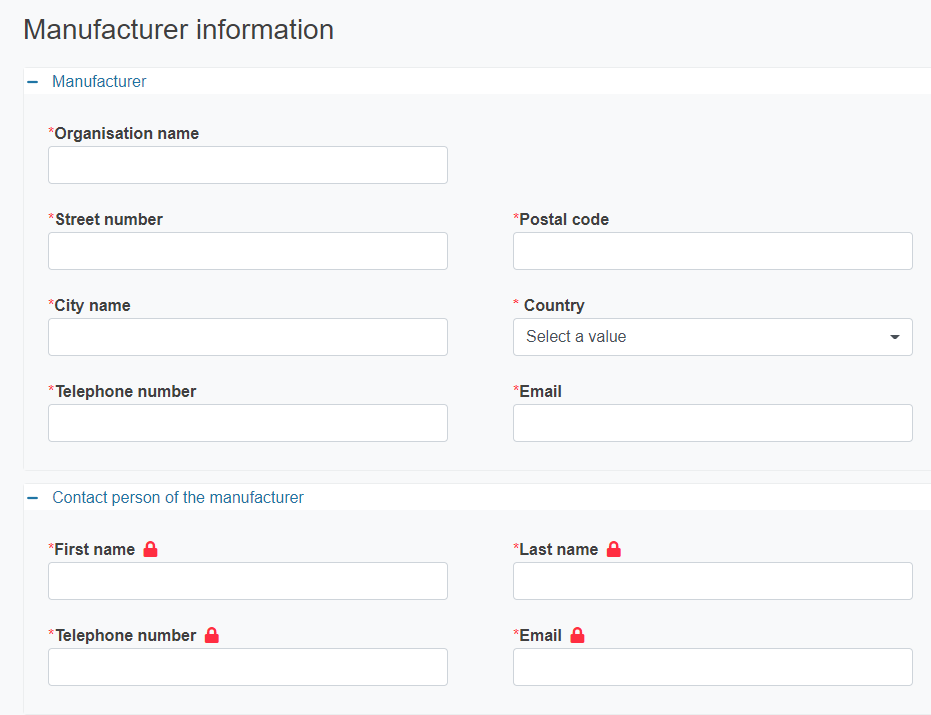
If the manufacturer is not registered in EUDAMED you must provide its details. The details of the contact person will not be made publicly available.
Below you can find a summary table where you can check the data you need to provide depending on certain conditions.
Do I need to provide the following data? | The device is not CE marked - CI/PS only, option not applicable for PMCF/PMPF | The device is CE marked but is not registered in EUDAMED | The device is registered in EUDAMED | |
|---|---|---|---|---|
CI/PS | PMCF/PMPF | |||
UDI-DI/EUDAMED ID | Yes | Yes | ||
UDI-DI - not registered in EUDAMED | Yes | |||
Issuing entity | Yes | |||
I confirm that the CE-marked device will be used outside the scope of its CE mark | Yes, for CI/PS only | Yes | ||
I confirm that the CE-marked device will be used within the scope of its CE mark | Yes, for PMCF/PMPF only | Yes | ||
Investigational/Study device details | ||||
Device ID (like Model number / Version) | Yes | Yes | Yes | Yes |
Device name | Yes | Yes | Only if different from registered device data | |
Device trade name | Yes | Yes | Only if different from registered device data | |
EMDN nomenclature code and nomenclature text | Yes | Yes | Select the EMDN code of the registered device that applies to the current CI/PS | Select the EMDN code of the registered device that applies to the current PMCF/PMPF |
Risk Class | Yes | Yes | Yes | |
Device description | Yes | Yes | Yes | Yes |
Intended (clinical) purpose | Yes | Yes | Yes | Yes |
Does the device contain or incorporate medicinal substance(s)? | Yes | Yes | ||
Does the device include human blood or plasma derivatives? | Yes | Yes | Yes | |
Does the device incorporate, as an integral part, or is it manufactured using non-viable biological substances? | Yes | Yes | Yes | Yes |
Has the device been subject to scientific views/an opinion from an Expert Panel and/or EURL? | Yes | Yes | Yes | Yes |
Manufacturer of the investigational/study device | ||||
Is the manufacturer the same as the sponsor? | Yes | Yes | Yes | Yes |
Is the manufacturer registered in EUDAMED | Yes | Yes | ||
You need to provide the authorised representative details only if the manufacturer is registered in EUDAMED and the country of the manufacturer is non-EU.