Comparator
Click Comparator from the left menu.
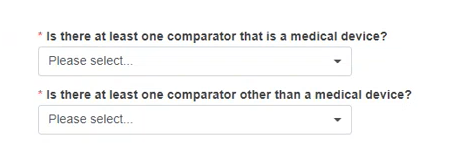
Indicate if there is at least one Comparator that is a medical device and if there is at least one Comparator other than a medical device, by selecting Yes or No.
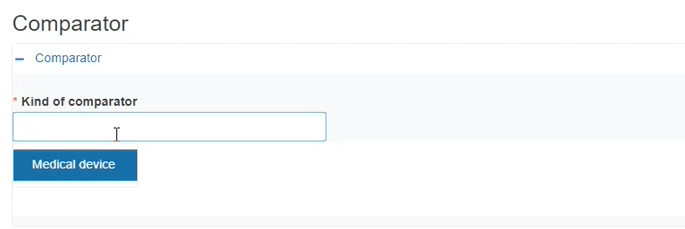
If you reply Yes to the first question, a new sub-section will appear for you to choose the type of Comparator device, which depends on the legislation selected in the first step.
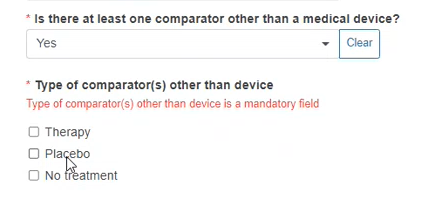
If you reply Yes to the second question, the system will display a list for you to choose the type of Comparator. You can select more than one option.
The following Comparators require further information:
Medical device
IVD medical device
IVD medical device according to Art 5(5) of the IVDR
To fill in the information of the above Comparators, follow the explanation presented in the chapter Investigational/Study device(s).
Note
For each Comparator device, the system generates a new sub-section under the Language-specific Comparator device(s) information header of the National Information section with the same identifier as the Comparator.
Below you can find a summary table where you can check the data you need to provide depending on certain conditions.
Do I need to provide the following data?
The medical device Comparator is not CE marked - CI/PS only, option not applicable for PMCF/PMPF
The medical device Comparator is CE marked but is not registered in EUDAMED
The medical device Comparator is registered in EUDAMED
CI/PS
PMCF/PMPF
UDI-DI / EUDAMED ID
Yes
Yes
UDI-DI - not registered in EUDAMED
Yes
Issuing entity
Yes
I confirm that the CE-marked device will be used outside the scope of its CE mark
Yes (PMCF/PMPF)
Yes
Will the CE-marked Comparator medical device be used in the clinical investigation/performance study within the scope of its CE mark?
Yes (CI/PS)
Yes
Investigational/Study device details
Device ID (like Model number / Version)
Yes
Yes
Device name
Yes
Yes
Device trade name
Yes
Yes
EMDN nomenclature code and nomenclature text
Risk Class
Yes
Yes
Device description
Yes
Yes
Yes
Yes
Intended (clinical) purpose
Yes
Yes
Yes
Yes
Does the device contain or incorporate medicinal substance(s)?
Yes
Yes
Does the device include human blood or plasma derivatives?
Yes
Yes
Does the device incorporate, as an integral part, or is it manufactured using non-viable biological substances?
Yes
Yes
Has the device been subject to scientific views/an opinion from an Expert Panel and/or EURL?
Yes
Yes
Manufacturer of the investigational/study device
Is the manufacturer the same as the sponsor?
Yes
Yes
Is the manufacturer registered in EUDAMED
Yes
Yes
Manufacturer Actor ID / SRN or Manufacturer details
Yes
Yes