General description
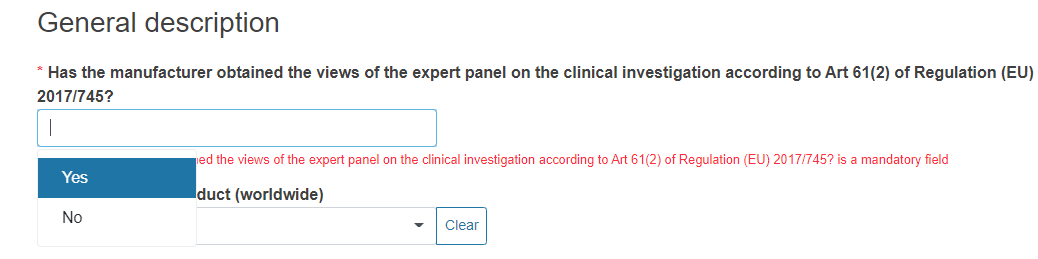
Reply to the question Has the manufacturer obtained the views of the expert panel on the clinical investigation according to Art 61(2) of Regulation (EU) 2017/745? by selecting Yes or No from the drop-down list.
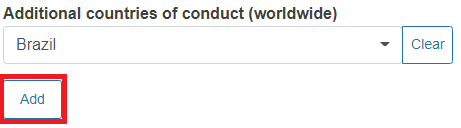
Add more countries of conduct, if relevant.
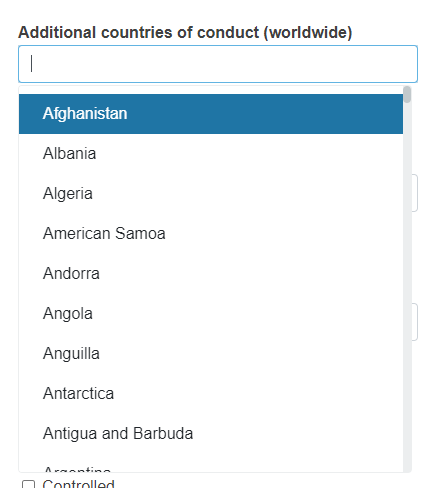
You can add several countries. To do it click Add and select the country from the drop-down list.
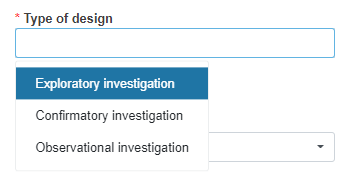
Select the type of design. At least one option must be selected.
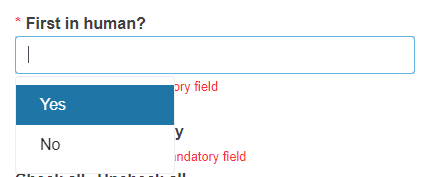
Reply Yes or No to the question First in human?
Select the design methodology. At least one option must be selected.
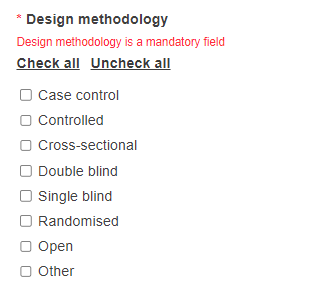
If you select the option Other you will be required to provide further information.
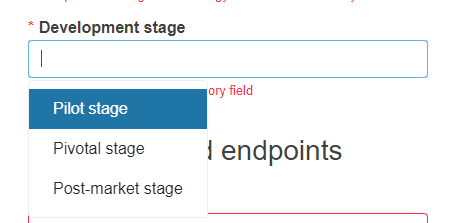
Select the development stage from the drop-down list. You can select only one option.