Add a device
Is the Basic UDI-DI known?:
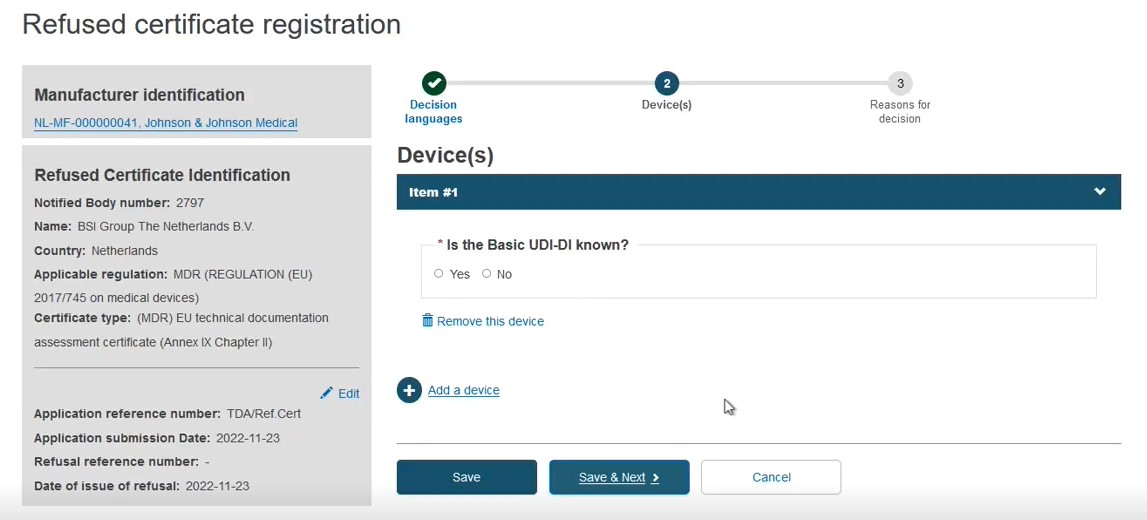 |
CASE A: The Basic UDI-DI is known
Select Yes in the field Is the Basic UDI-DI known?:

Enter part/all of the Basic UDI-DI code and click on the Check registry button. Only devices that are eligible for this type of procedure or certificate, and only those related to the manufacturer, will appear.
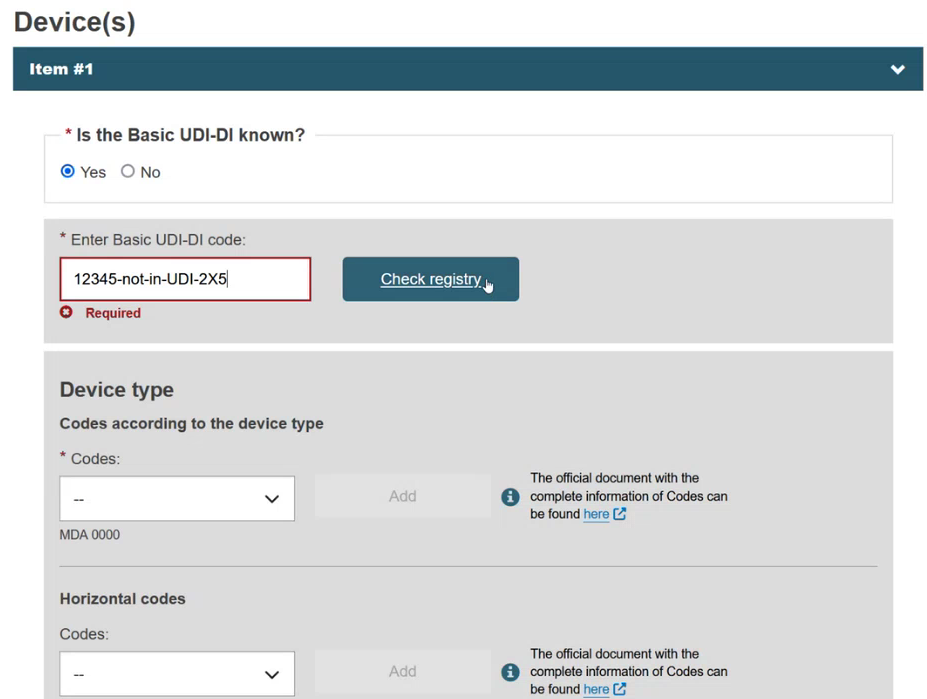
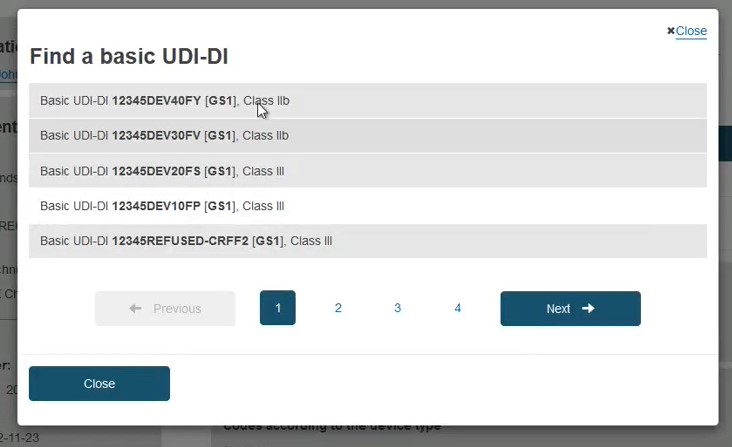
If the device is already registered in EUDAMED, then select the device from the list:
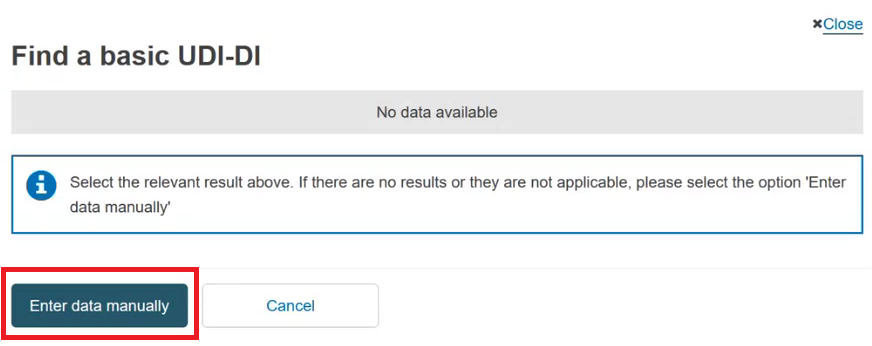
Otherwise, if the device is not yet registered in EUDAMED, click on the Enter data manually button:
Select the Issuing Agency and the risk class of the device and specify if the device is implantable or not (property applicable only for MDR):
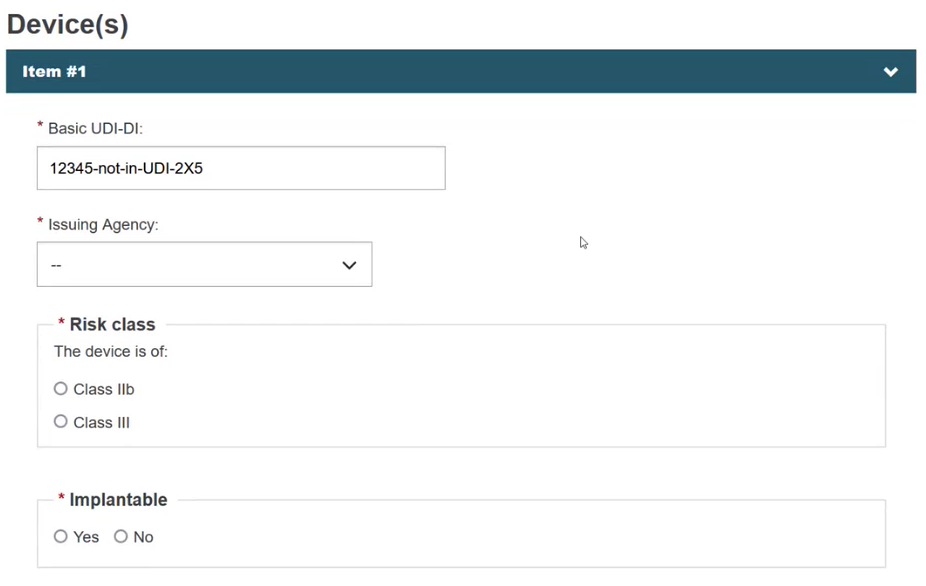
Specify if the device model is applicable or not:
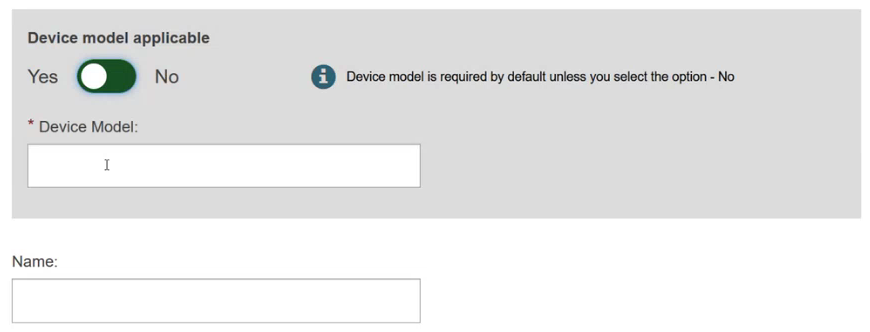
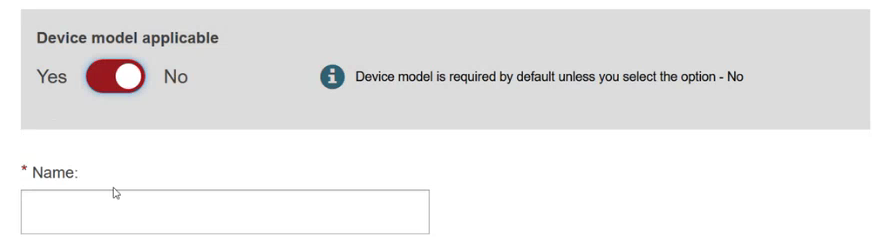
Add some characteristics of the device:
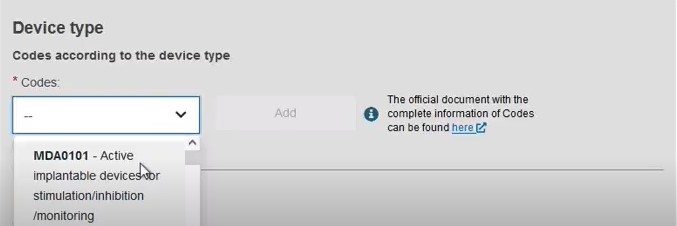
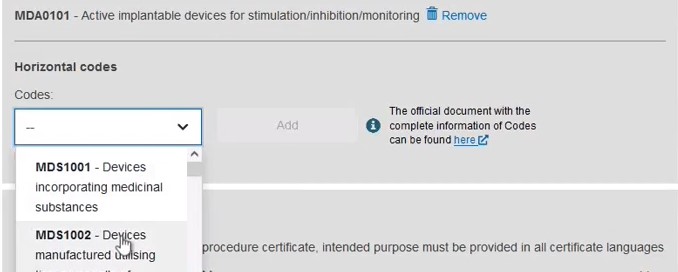
Add a description of the purpose:
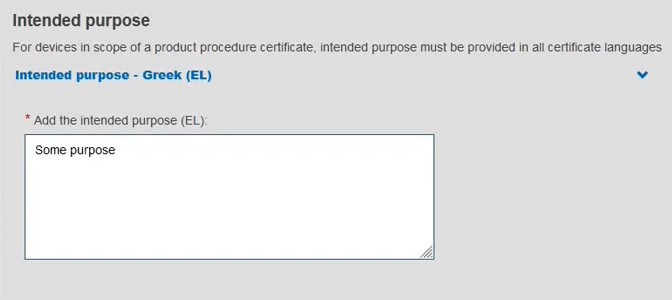
Click Save or Save and Next.
For additional devices, click + Add a device.
CASE B: The Basic UDI-DI is not known
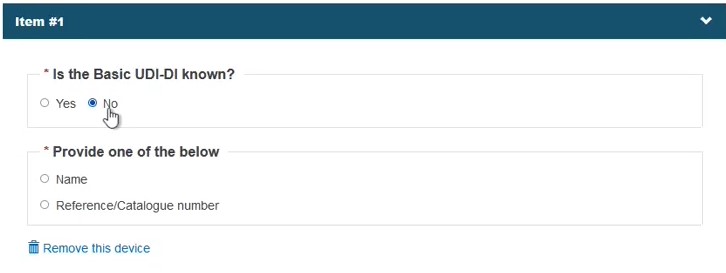
Select No in the field Is the Basic UDI-DI known?:

Identify the device by Name or alternatively, provide the Reference/Catalogue number in free-text and select the Risk Class. Note the Name or Reference/Catalogue number options also apply when registering Refused/withdrawn applications:
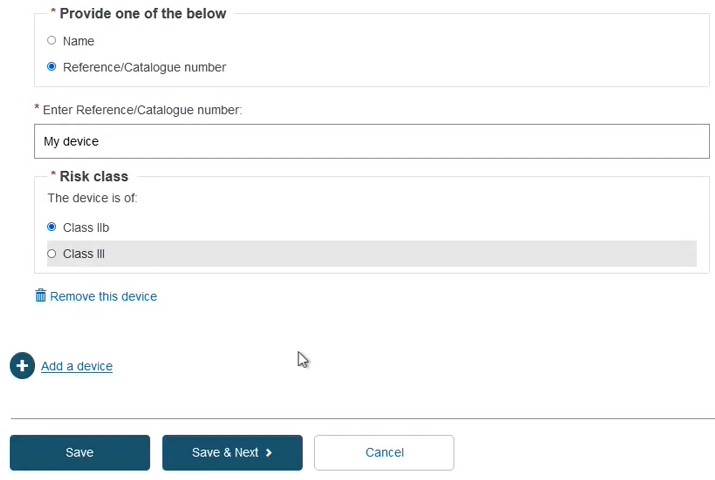
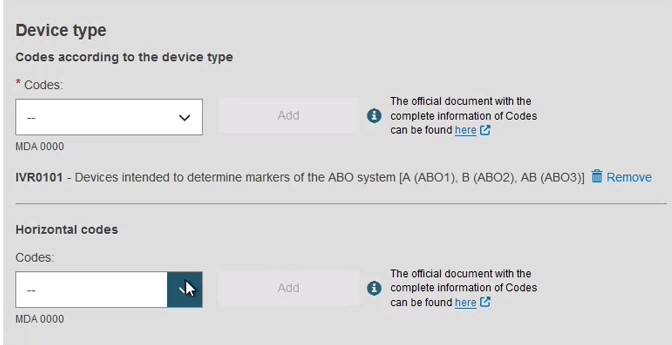
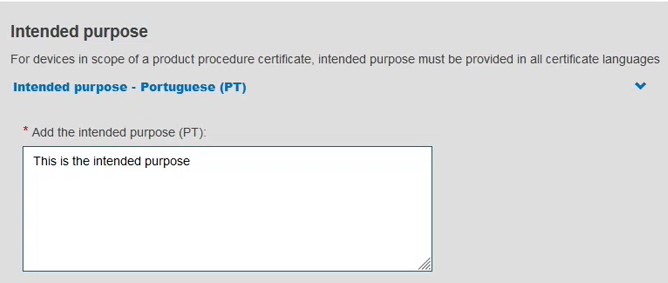
Provide the device type and the intended purpose:
Click Save or Save and Next.
For additional devices, click + Add a device.