Re-issuing a Quality/Product certificate
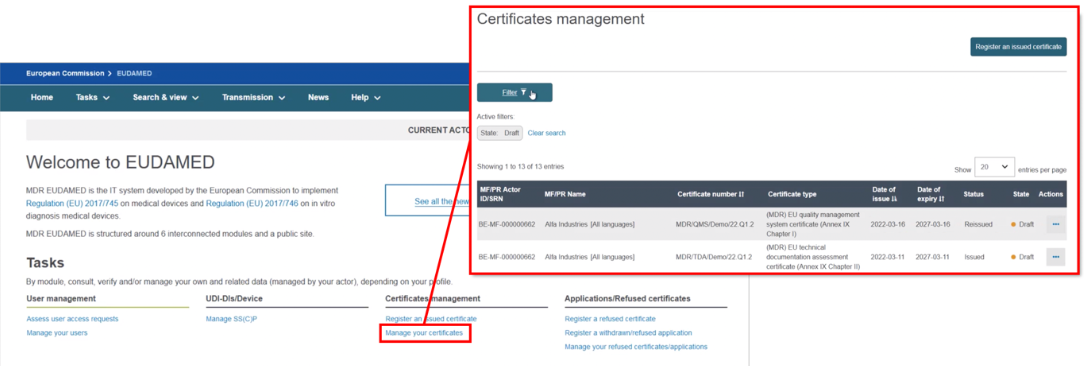
To re-issue a certificate, for example due to its imminent expiry, click on the Certificates management page then filter to identify the certificate you want to re-issue:
Warning
Notified Bodies may have up to three months between expiry date of previous certificate and the issued date and starting validity date of re-issued certificate.
Select Registered as the state. From the list generated, in the Actions menu click the three dots next to the intended issued certificate and select Re-issue Certificate:
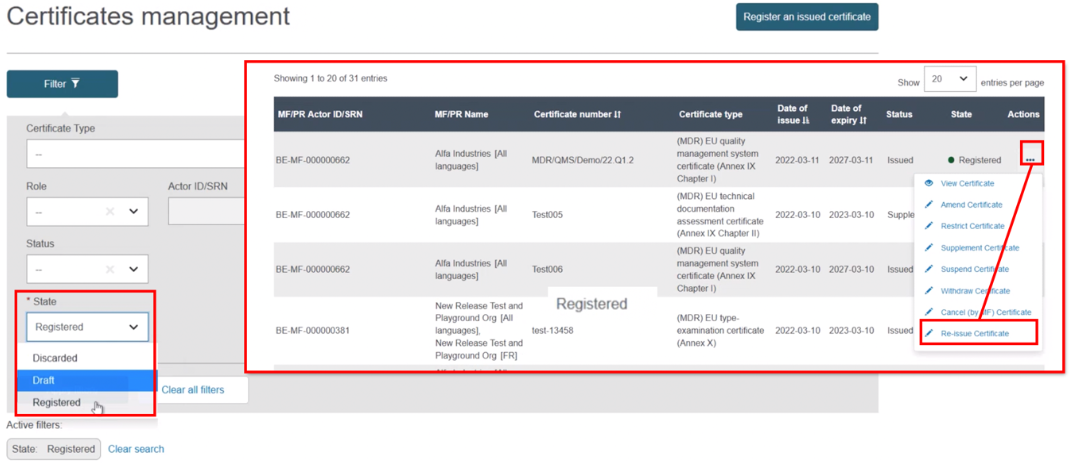
The next screen will display all relevant information of the certificate. If necessary, click Update with new actor version:
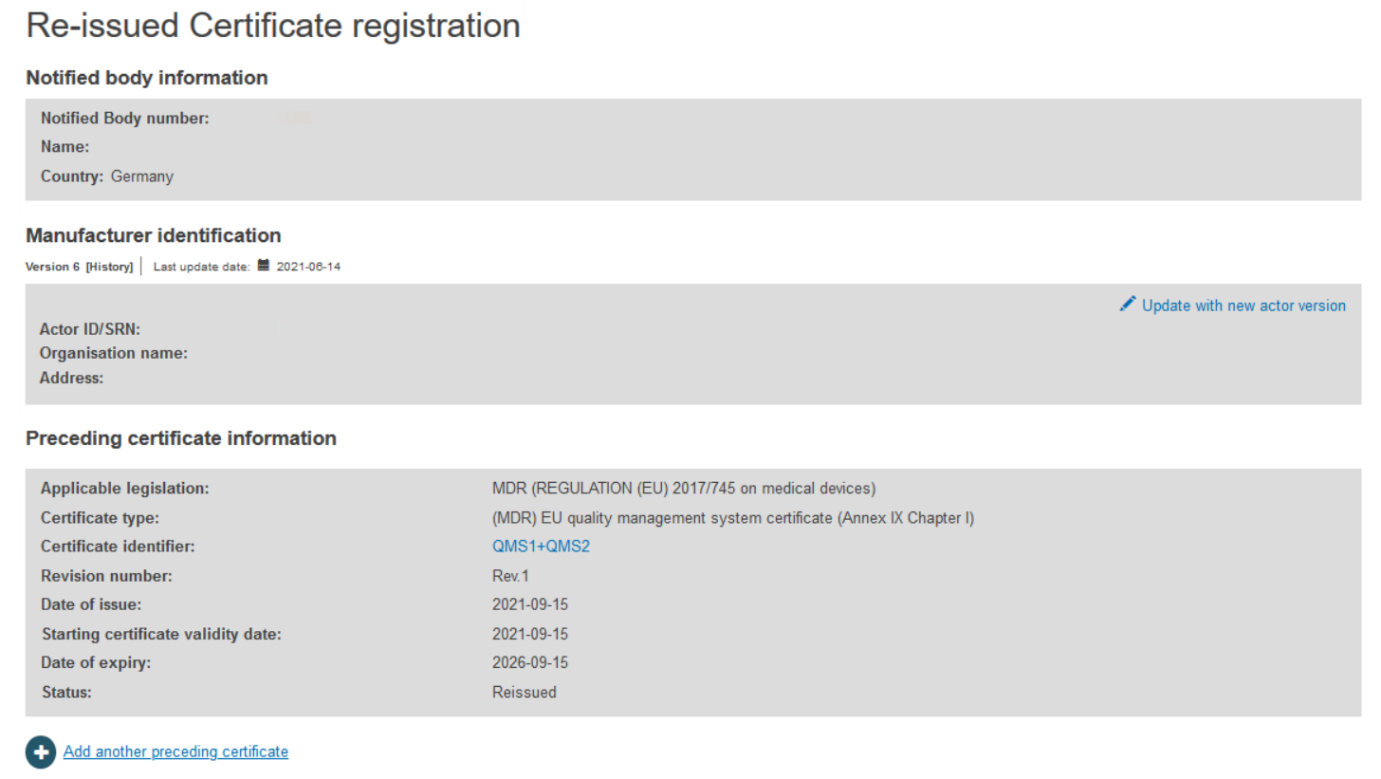
Select the right actor version from the list:
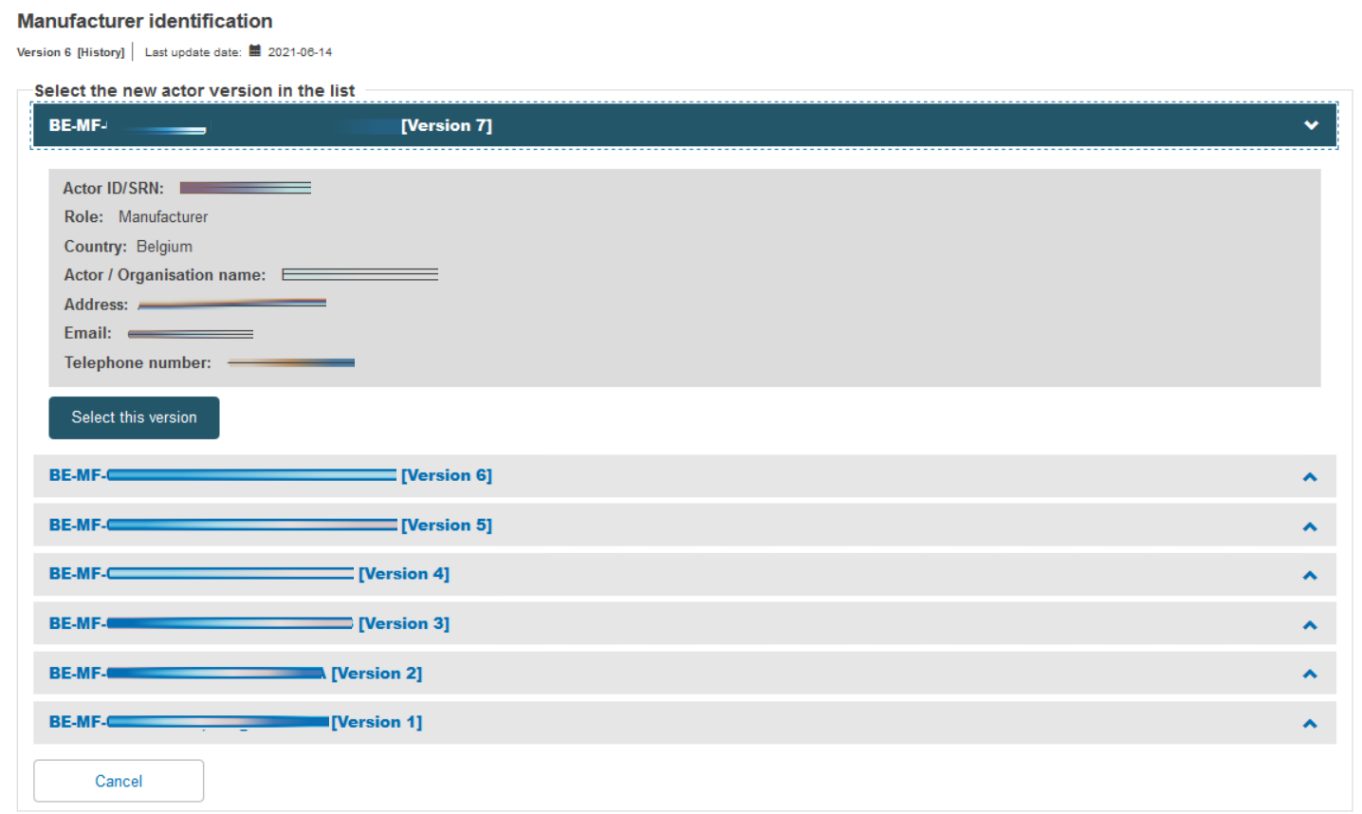
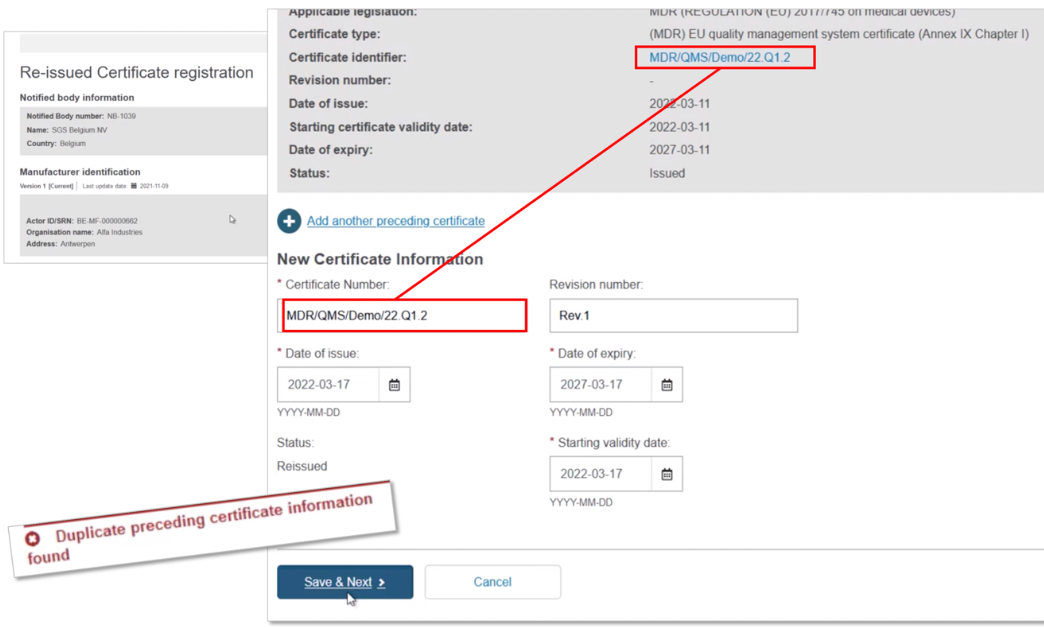
Scroll down to the New Certificate Information section. Duplicate the certificate identifier, and note the duplication warning message. Add a Revision number so it differs from the preceding certificate – the warning disappears. Select the new issue date, validity date and expiry date (noting the maximum period is five years). Now click on Save & Next to proceed:
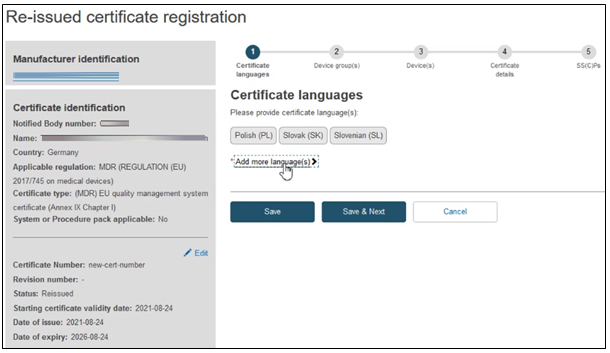
The next screen will display a timeline of steps to follow. Follow the order, starting from the first section Certificate languages.
Click on Add more languages if necessary:
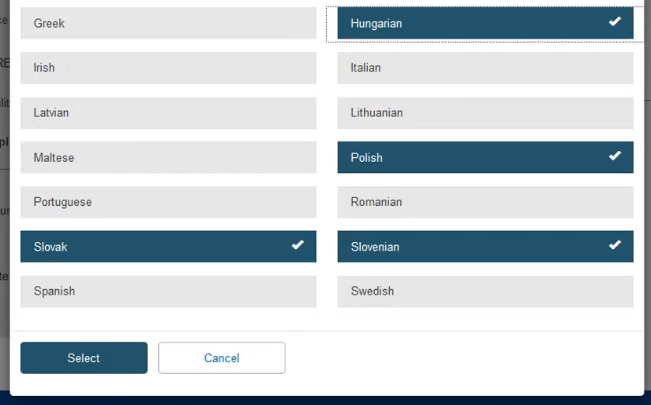
On the pop-up window, click on the desired languages and press Select:
Click Save & Next to proceed to the next section:
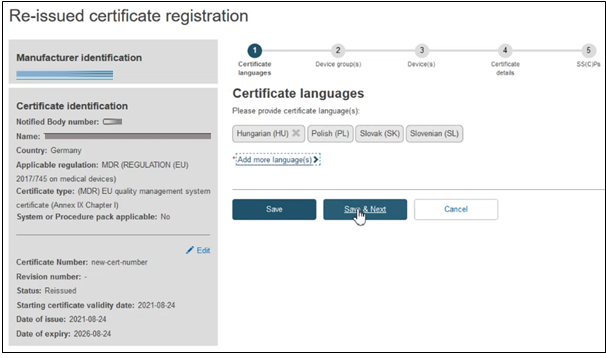
Fill in the information required to complete the Device group(s) step, when a certificate is re-issued, no change can be made.
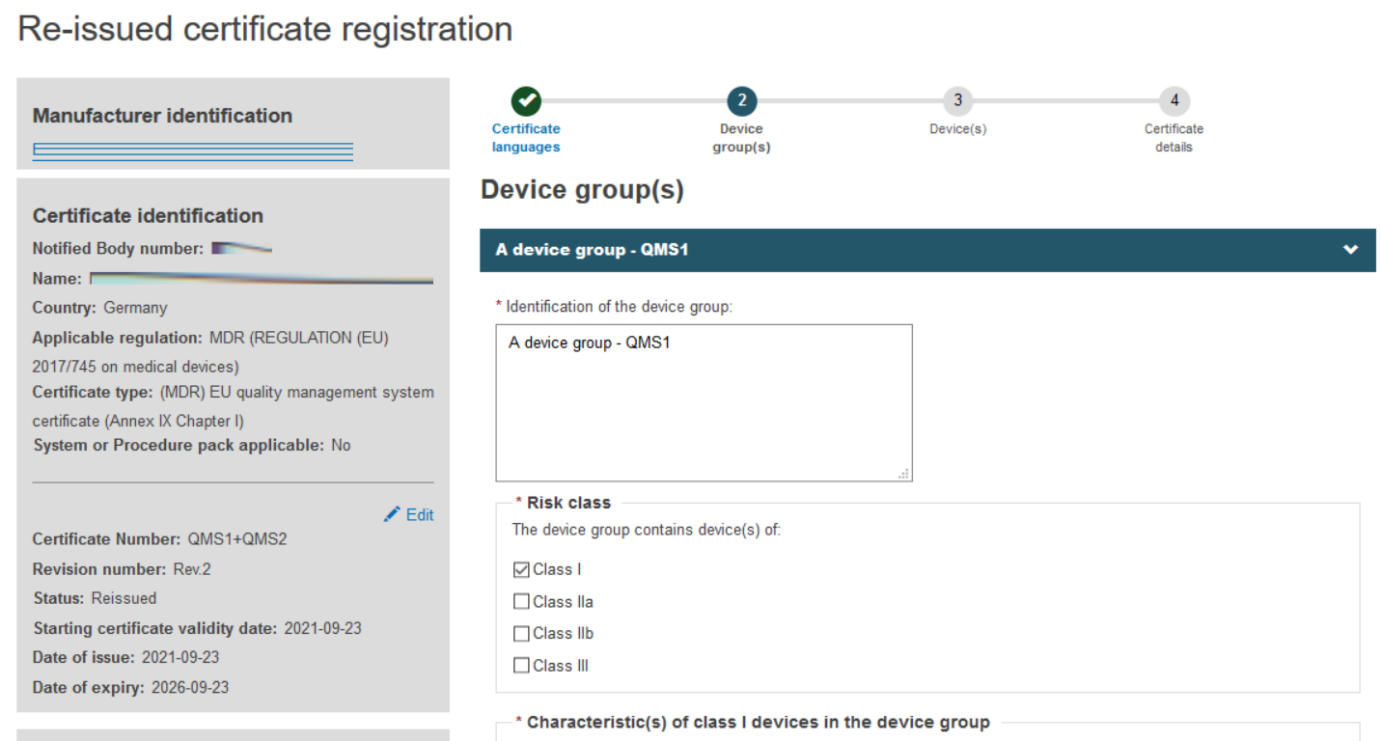
Click + Add a device group and then again on the appearing Device group item:
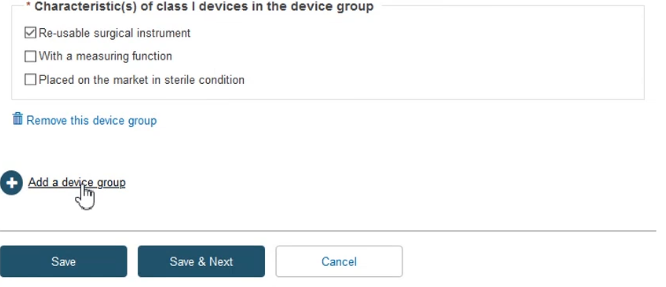
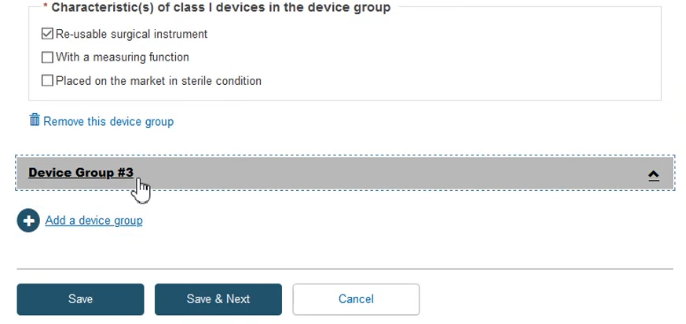
Fill in the required information and click Save & Next:
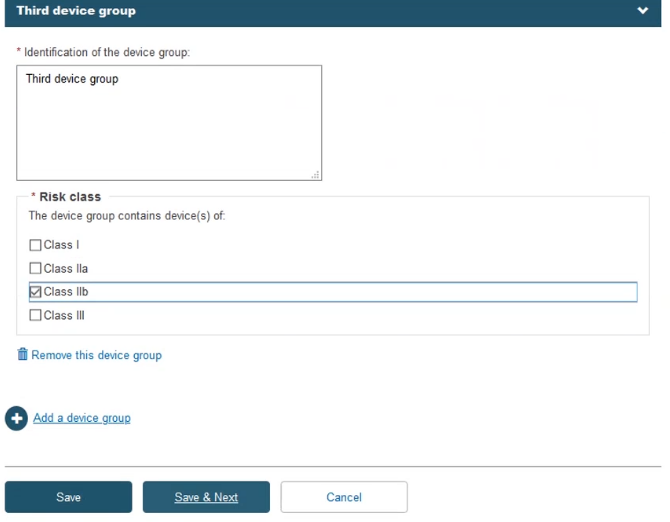
Fill in the information for the Device(s) step:
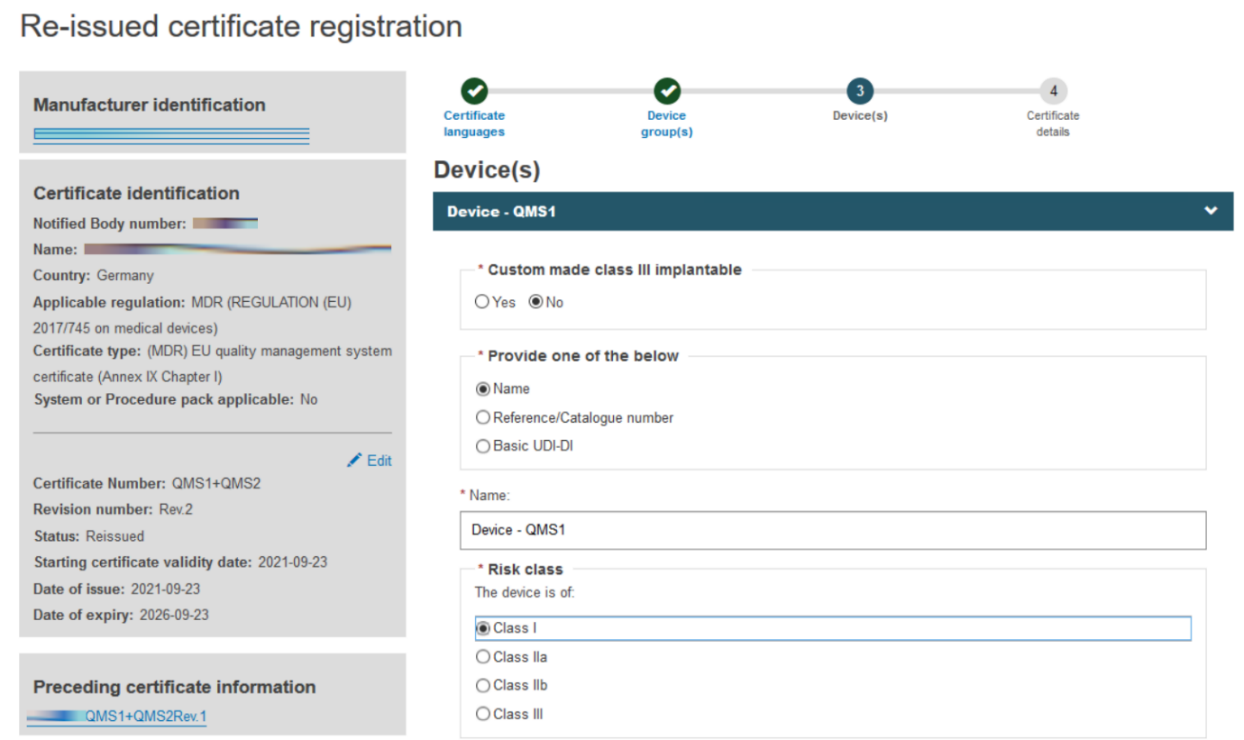
Click + Add a device group and then click on the Item that displays under Device(s):
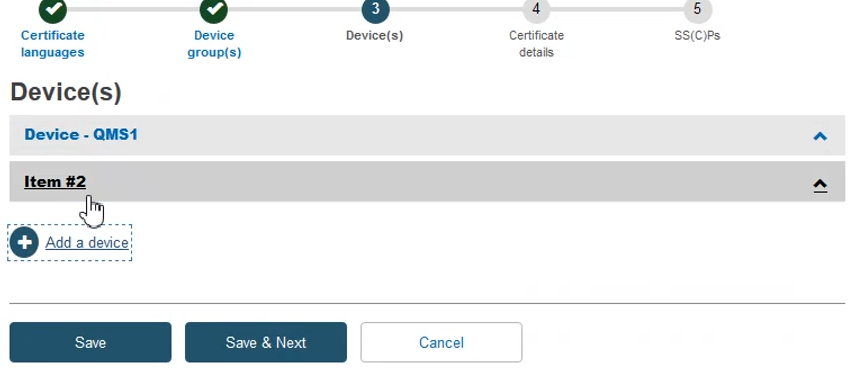
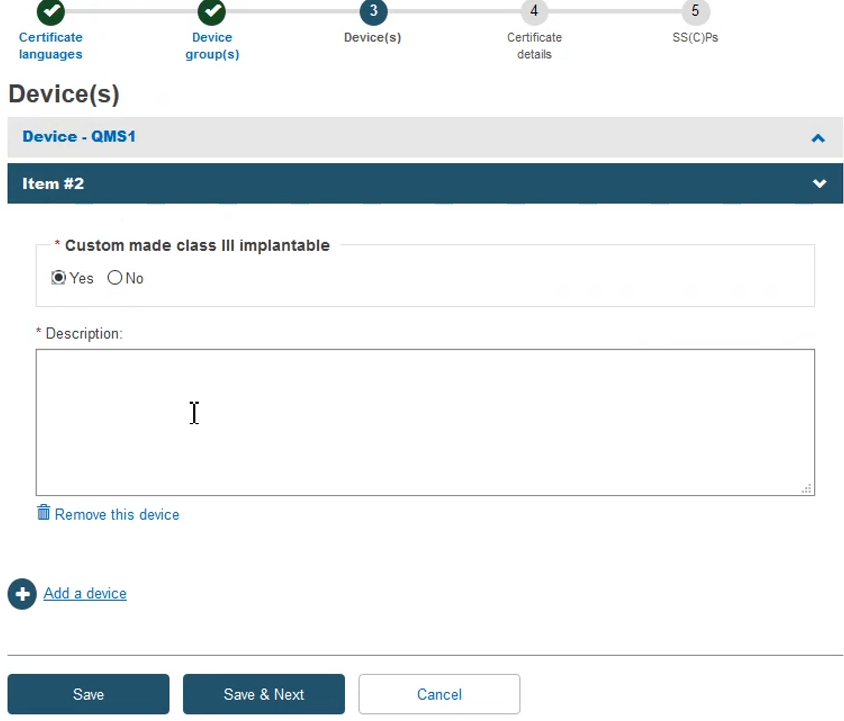
Add the required information to complete this step and then click Save & Next:
Fill in the information required to complete the Certificate details step:
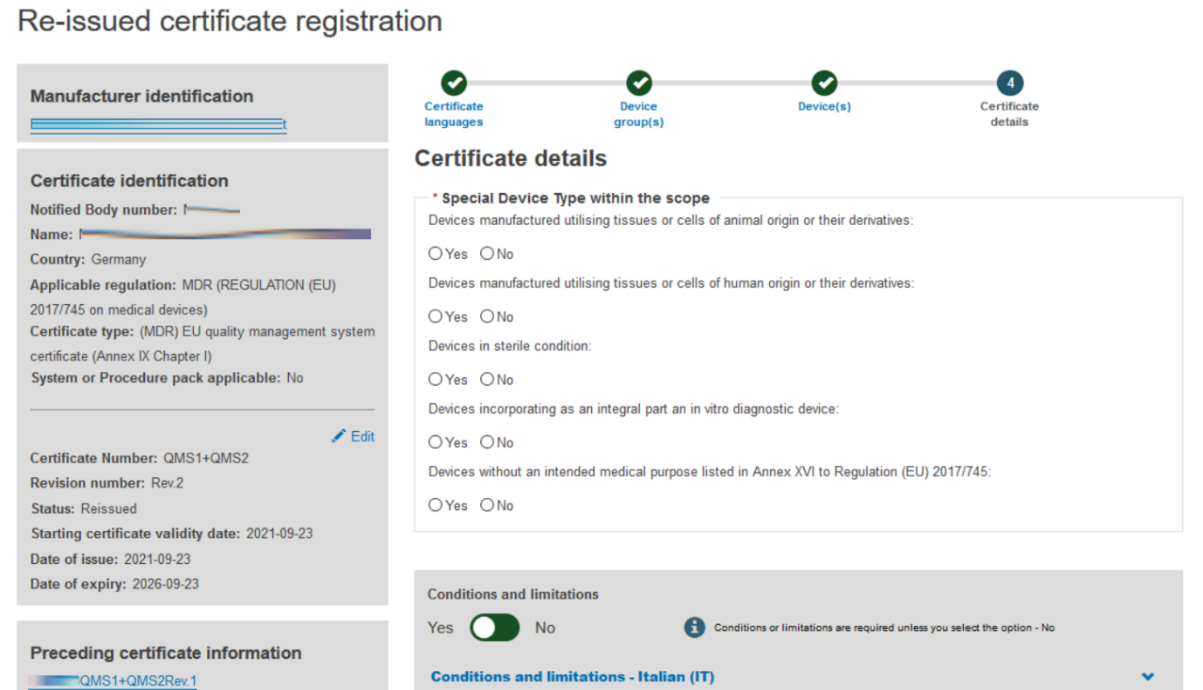
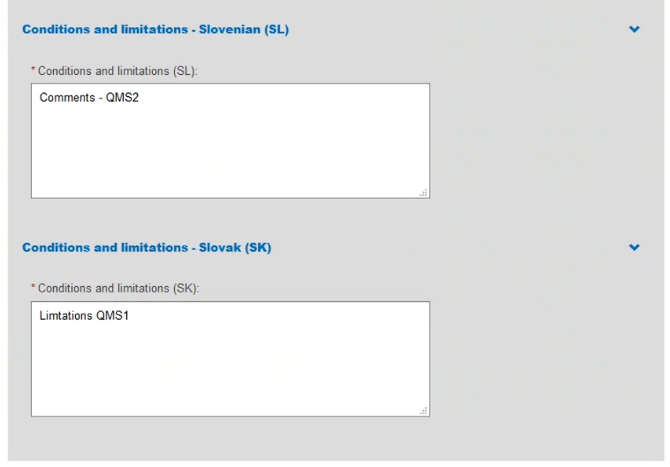
Provide comments regarding Conditions and Limitations in each language you selected:
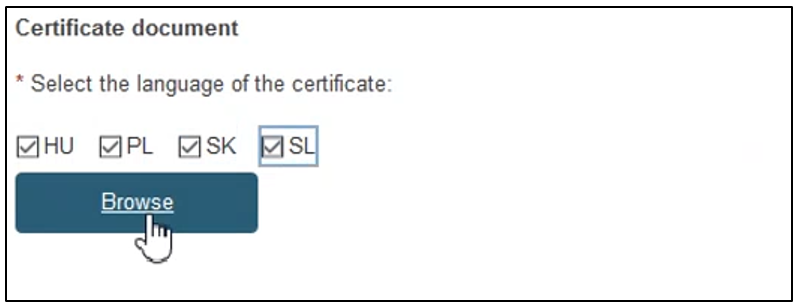
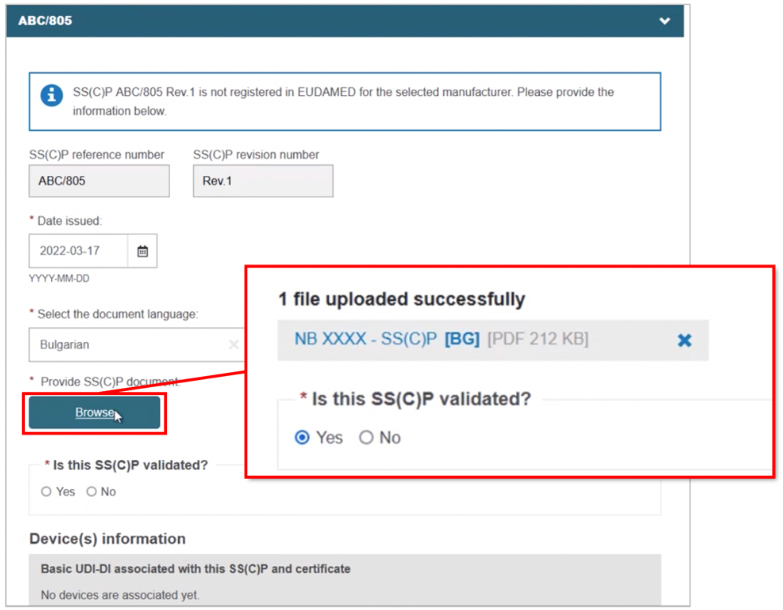
To provide the new re-issued certificate document, tick the relevant languages and click Browse to upload the document(s) from your computer. You can upload either one document per language or one document covering all languages:
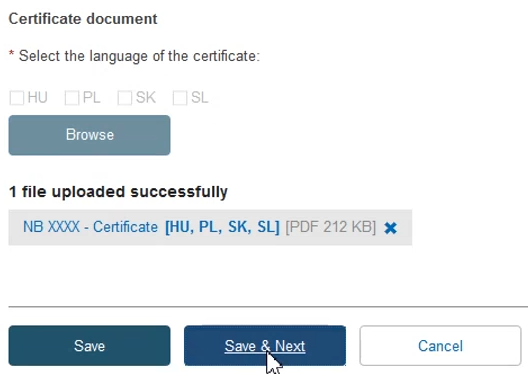
Once you have successfully uploaded the new certificate document(s), click Save & Next:
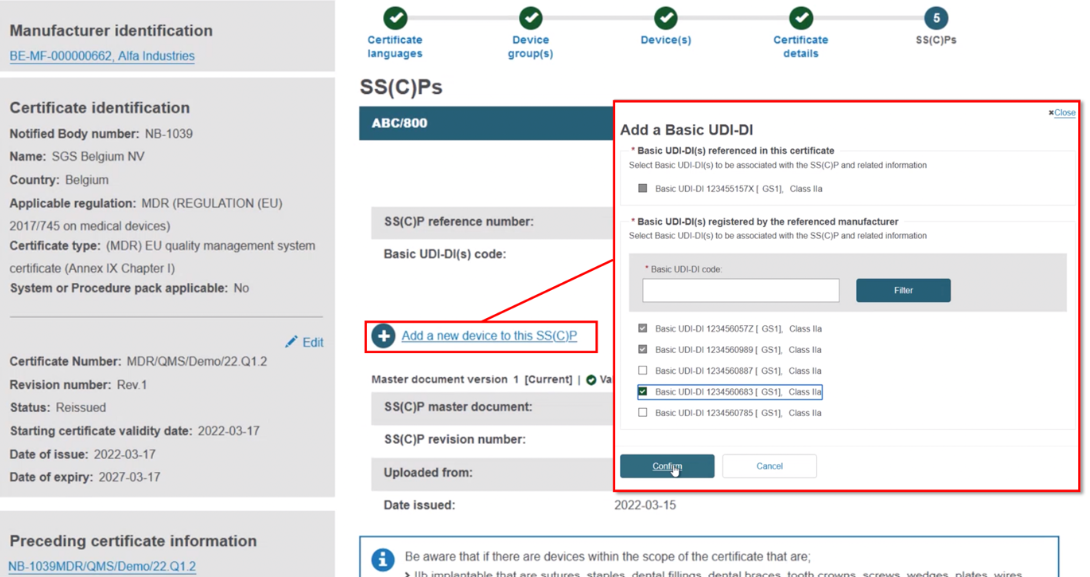
At this point, depending on the specifics of the certificate, the system may take you to the next step called SS(C)Ps. If not relevant for the specific certificate, this step will be omitted. You have three possibilities when adding new devices.
 Add device(s) to an existing SS(C)P from the preceding certificate (see Step 19).
Add device(s) to an existing SS(C)P from the preceding certificate (see Step 19). Add device(s) to a new version of the SS(C)P from the preceding certificate (see Step 20).
Add device(s) to a new version of the SS(C)P from the preceding certificate (see Step 20). Add device(s) to a newly registered SS(C)P (see Step 21).
Add device(s) to a newly registered SS(C)P (see Step 21).Add device(s) to an existing SS(C)P from the preceding certificate.
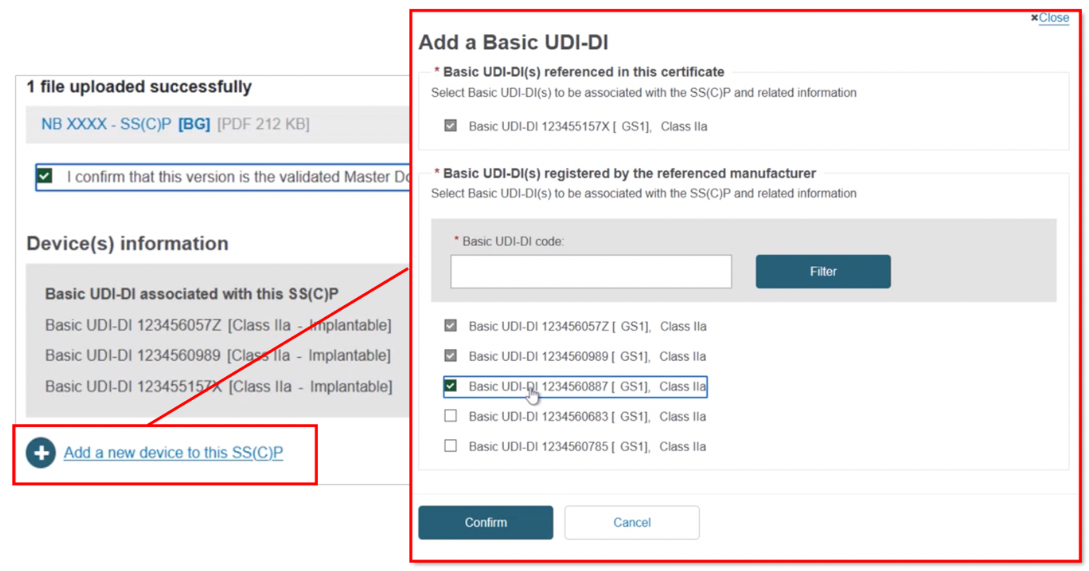
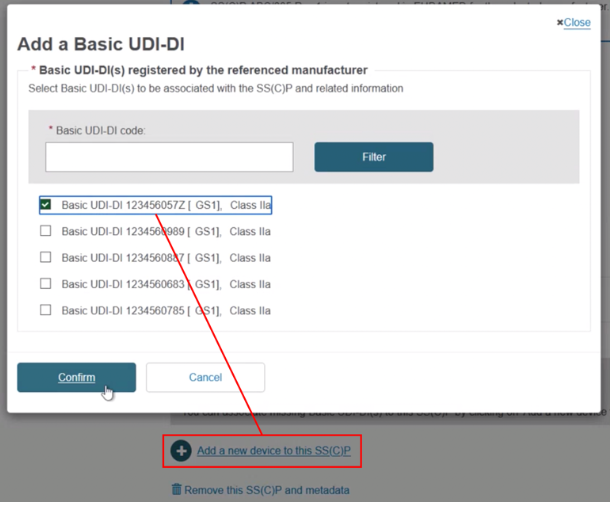
Click on Add SS(C)P. The existing devices show, but are inactive. Select any new device(s) and click Confirm:
Note
If you have referenced a Basic UDI-DI in Submitted state, then upon submitting your certificate, you will be asked to confirm the accuracy of the device data registered for the referenced Basic UDI-DI:
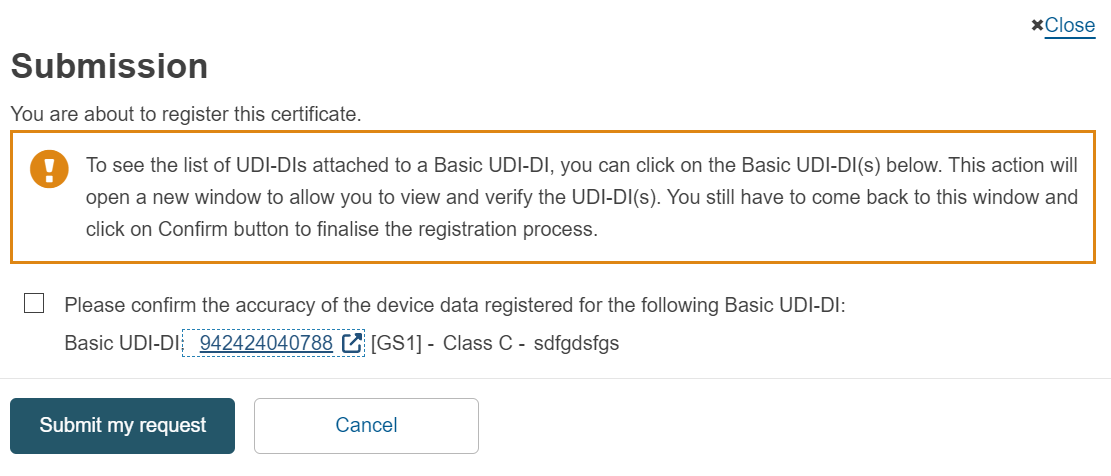
After the certificate is submitted, the state of the Basic UDI-DI will be updated to Registered. The Basic UDI-DI(s) and their related UDI-DI(s) will then become visible on the EUDAMED public site.
The new device appears next to the dustbin icon. If you save now, the new device will be linked to this version of the SS(C)P:
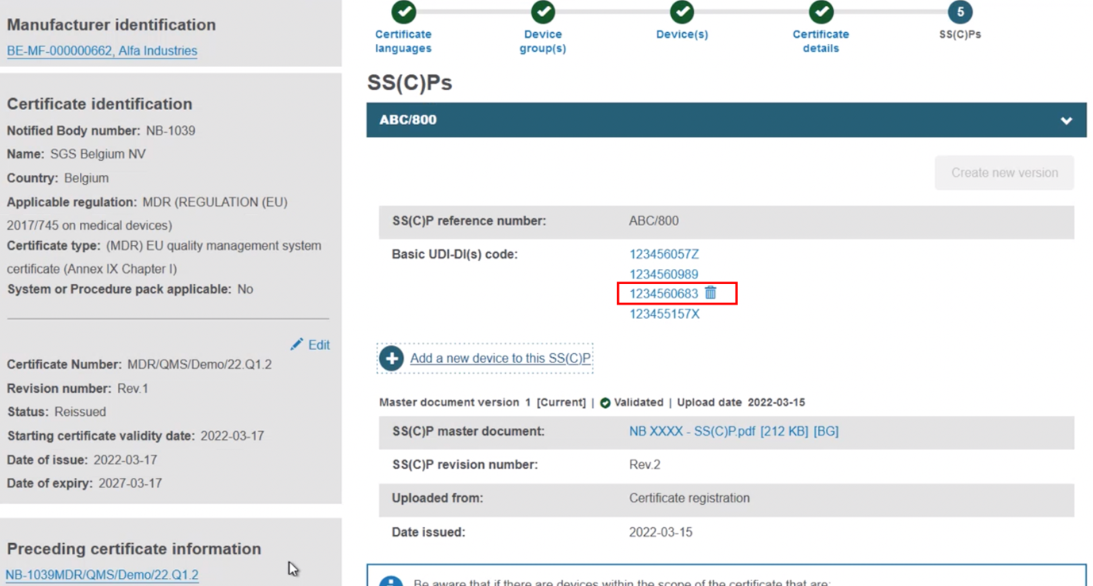
Add devices to a new version of the SS(C)P from the preceding certificate. With this approach, click Create new version:
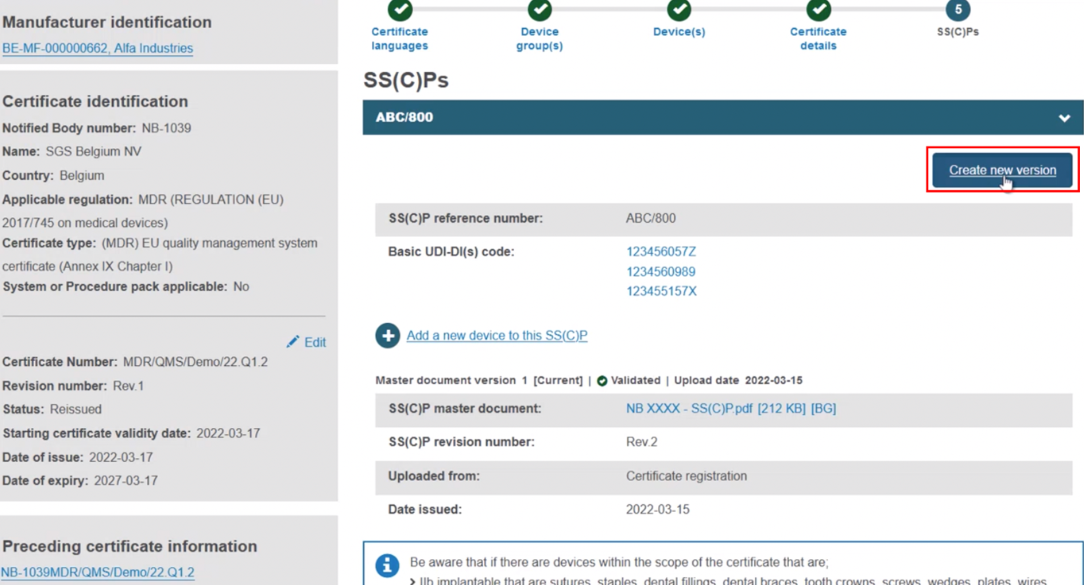
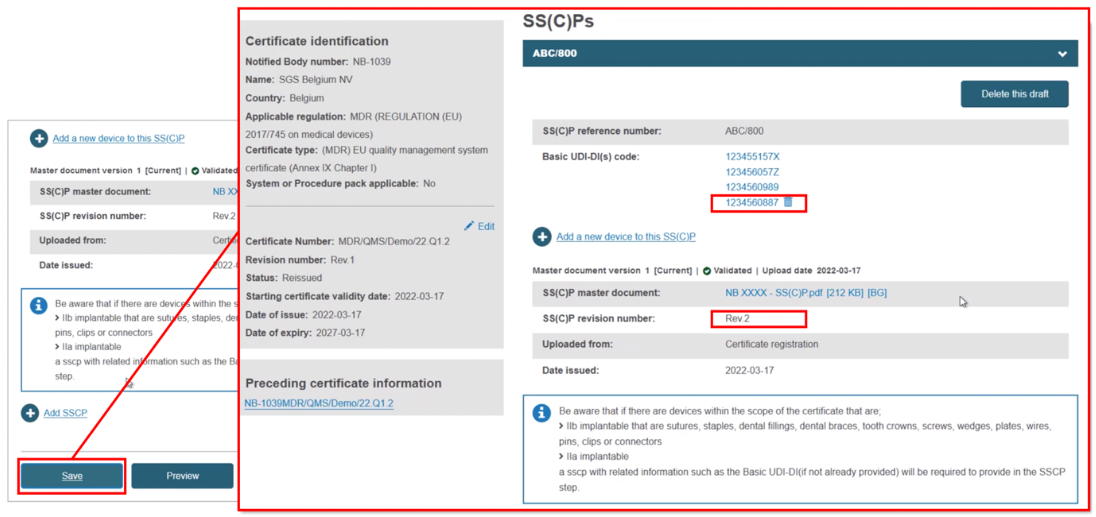
Input the SS(C)P reference number, and create a revision number, then specify the issue date:
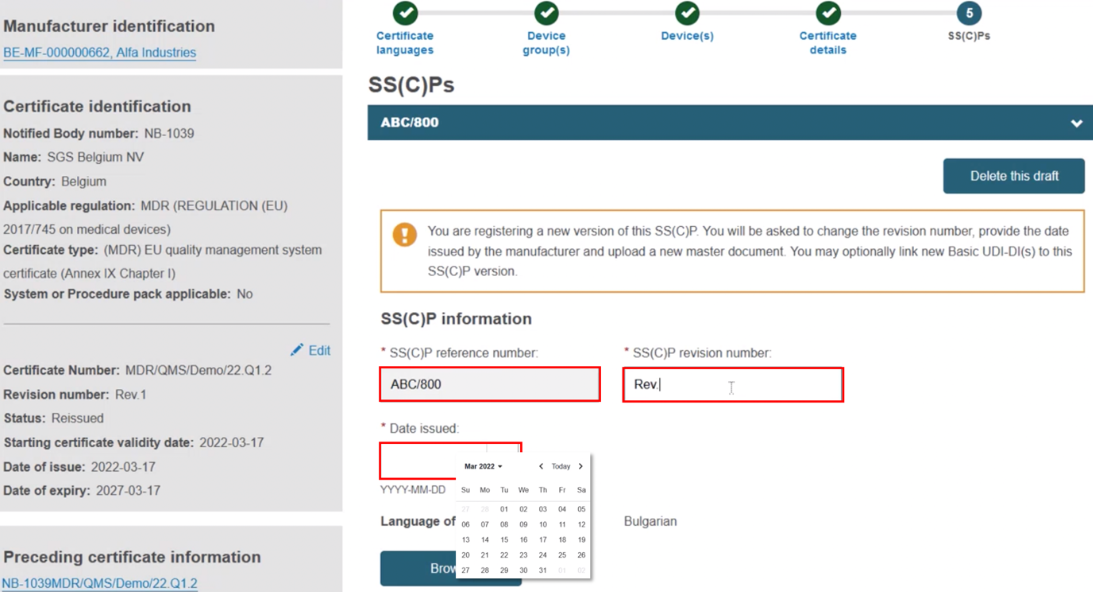
Click Browse to locate and upload the master document, and click to confirm it is validated:
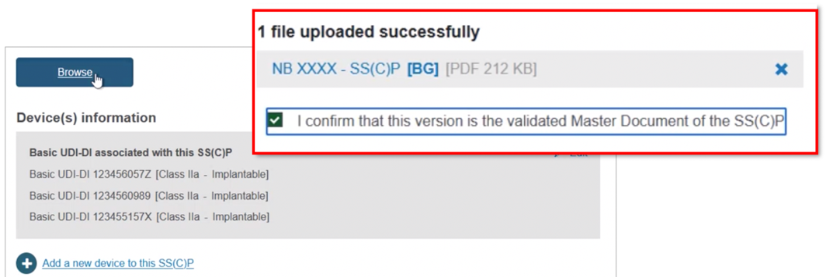
Click + Add a new device to this SS(C)P, locate and select the new device, and click Confirm to link it to this new SS(C)P version:
Note
If you have referenced a Basic UDI-DI in Submitted state, then upon submitting your certificate, you will be asked to confirm the accuracy of the device data registered for the referenced Basic UDI-DI:

After the certificate is submitted, the state of the Basic UDI-DI will be updated to Registered. The Basic UDI-DI(s) and their related UDI-DI(s) will then become visible on the EUDAMED public site.
Click Save, and when you register the certificate, this SS(C)P will be saved:
Registering a new SS(C)P, then adding device(s) to it. Click + Add SSCP, then provide the reference and revision number:
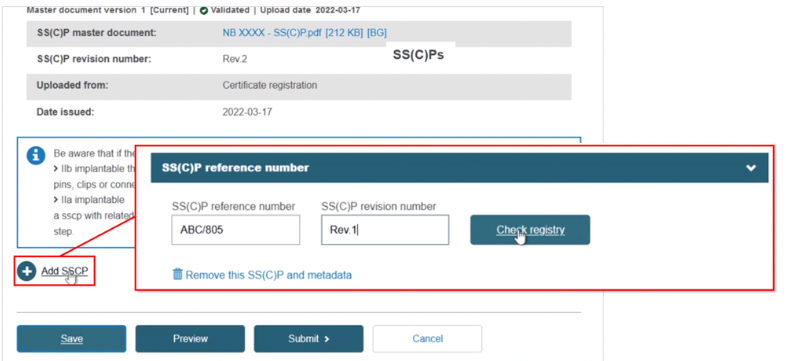
Click Check registry. The system will confirm this is a new SS(C)P:

Complete the fields for the new SS(C)P, including the master document language. Click Browse to locate and upload the master document, confirming it is validated (for Quality-type certificates):
Scroll to the bottom and click + Add a new device to this SS(C)P, then select the device(s). Click Confirm and Save:
At this point, if a high risk device, as defined in Article 55 (MDR) and Article 50 (IVDR), was referenced, the system will take you to the next step called Mechanism for scrutiny.
If the certificate you are reissuing is associated with an IVDR high risk device, the fields appearing in the Mechanism for scrutiny step will be the same as in the Case B of the Provision of Mechanism for scrutiny page. Otherwise, if the certificate you are reissuing is associated with an MDR high risk device, follow the steps listed below.
Case A: CECP records found in the system
Follow the steps described in the Case A of the Provision of Mechanism for scrutiny page.
Case B: No CECP records found in the system
Select Yes or No in the field Was CECP followed?:
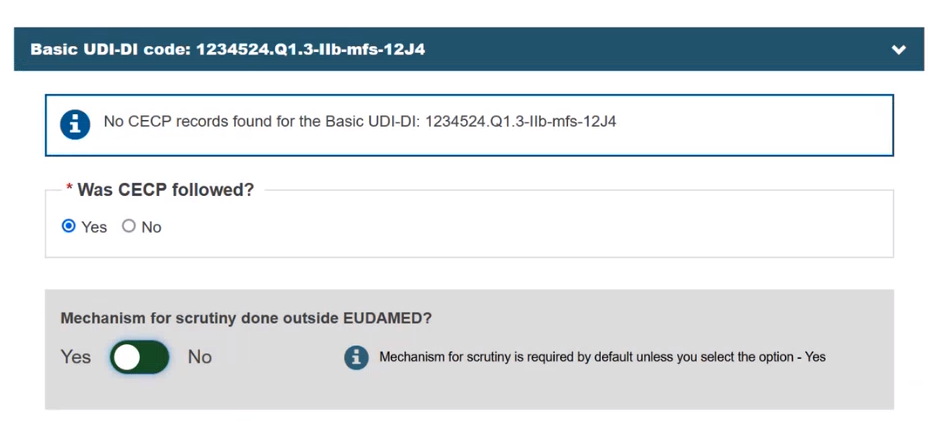
If you select Yes, the Mechanism for scrutiny done outside EUDAMED? field appears and you must set the toggle button to Yes to indicate that the Mechanism for scrutiny was done outside EUDAMED. Otherwise, the system will prevent you from continuing, unless you have selected No in the Was CECP followed? field.
Note
It is also possible to scrutinise again a device for which the initial Mechanism for scrutiny has been finalised. If a new CECP record has been registered for the Basic UDI-DI then, during the certificate reissue process, the Mechanism for scrutiny step will be provided.
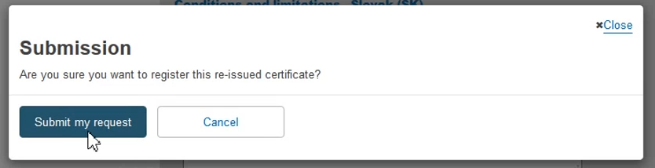
After having reviewed all information, click Submit:

The system will prompt you to confirm your submission of a re-issued certificate.
Click Submit my request to finalise the process:
The system will confirm your submission has been successful. You can also view the newly created certificate by clicking on the link provided:
Merging two or more certificates when re-issuing a Quality certificate