PSR information
Under the PSR information tab, select one or more PSR types:
Note
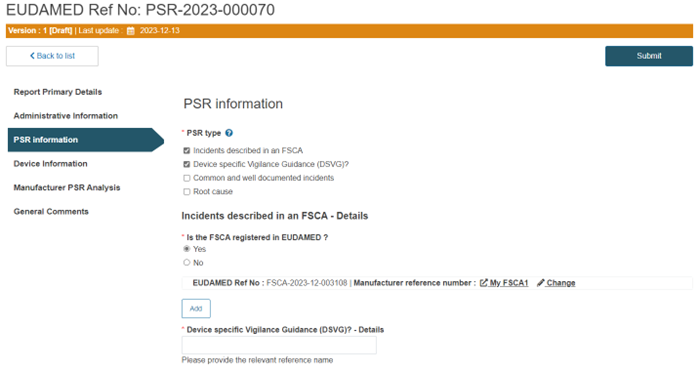
If the PSR type is related to incidents described in an FSCA, you have to additionally specify if the FSCA is registered in EUDAMED:

Similarly, if the PSR type is Device-specific Vigilance Guidance, you must insert the relevant reference name in the mandatory field Device specific Vigilance Guidance (DSVG)? - Details:
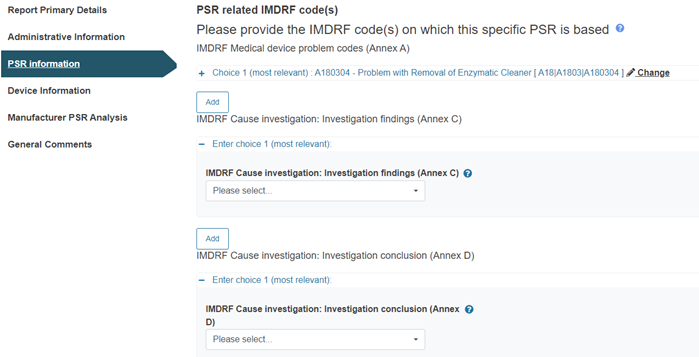
Provide at least one IMDRF code relating to the PSR in each of the three Annexes provided:
Next, provide the frequency of reporting for the PSR analysis update (the default value is three months):
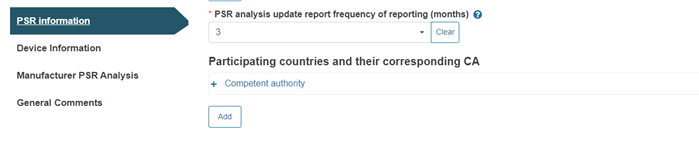
Note
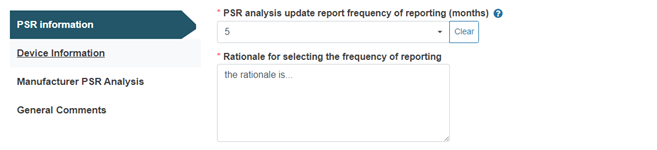
If the value selected is other than the default three months, a rationale must be provided:
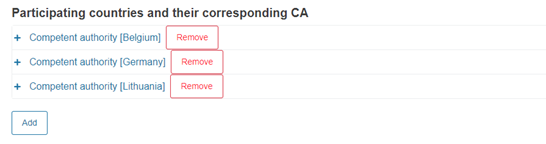
Finally, add other participating countries and their CAs:
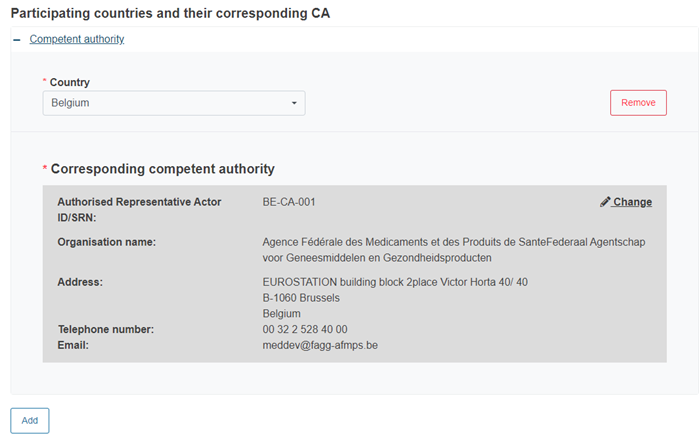
Click Add to add more countries and their CAs, if needed: