Device information
Under the Device information section, start by selecting the regulatory scope type:
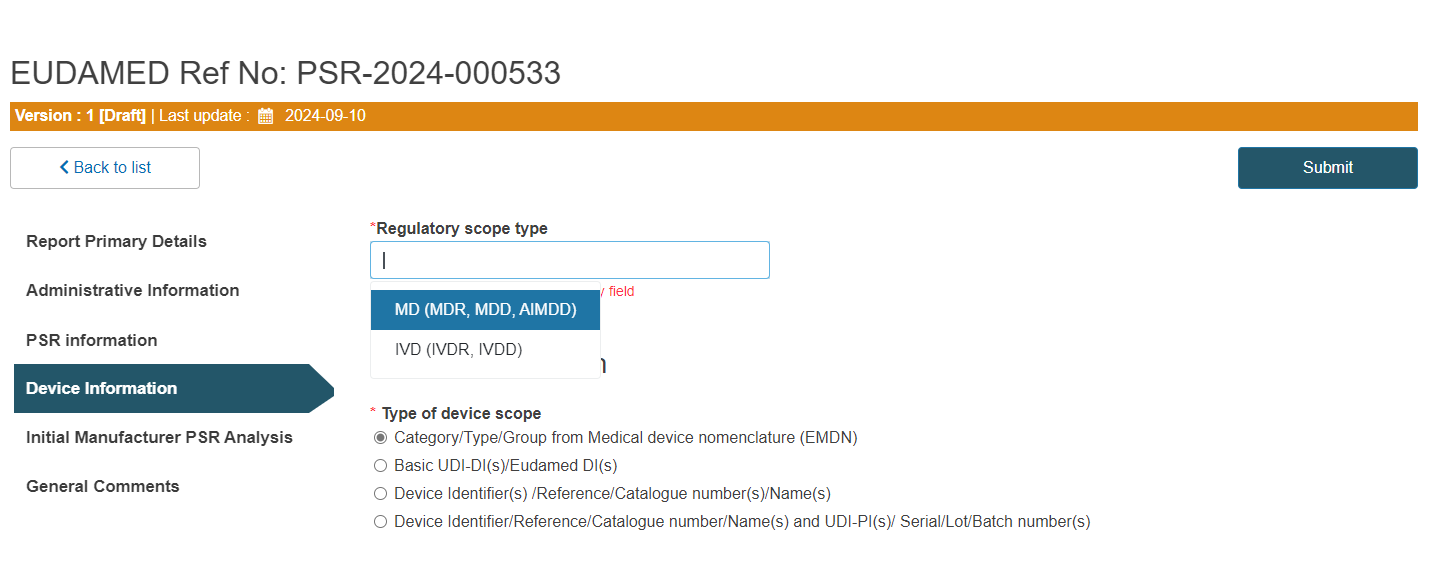
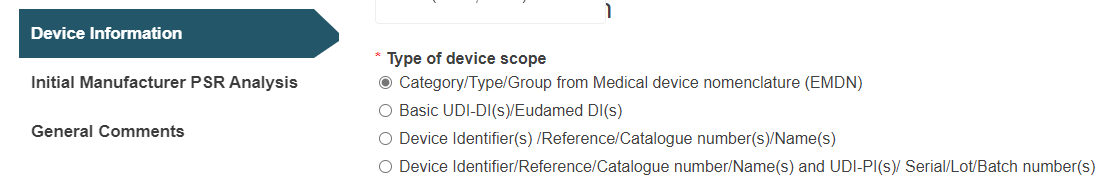
Select the type of device scope:
Note
Please note that in case of a special device type (requiring a Master UDI-DI), the GTIN field will appear for you to fill in:

Follow Option A, B, C or D
A. Category/Type/Group from Medical device nomenclature (EMDN):
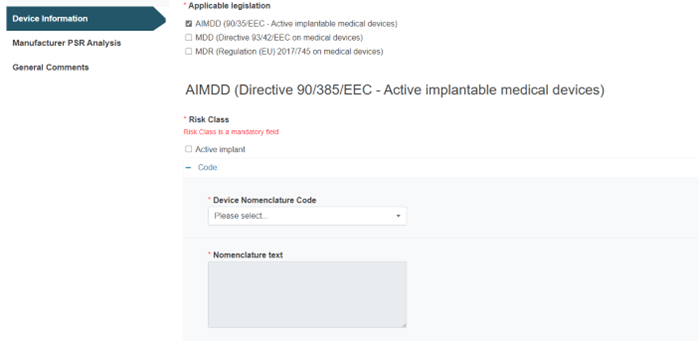
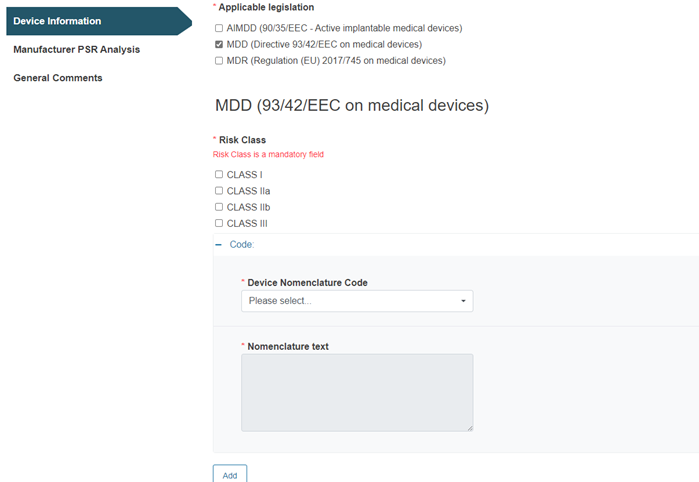
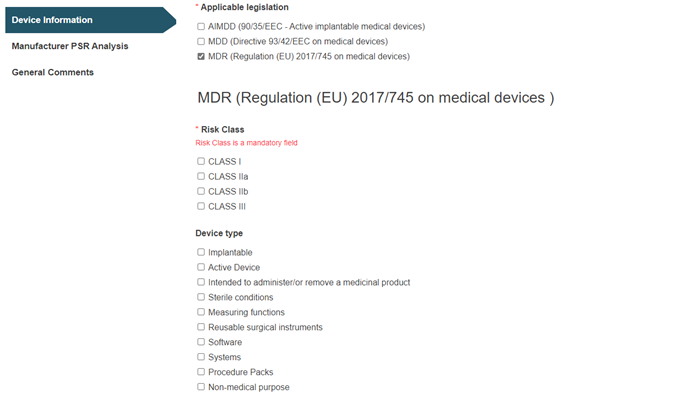
For the Category/Type/Group from Medical device nomenclature (EMDN) option, you must select the applicable legislation(s):

Depending on the applicable legislation(-s) selected, different Risk Class and Device type values will be displayed.
Fill in the fields Device Nomenclature Code and Nomenclature text accordingly:
Example with AIMDD as applicable legislation:
Example with MDD as applicable legislation:
Example with MDR as applicable legislation:
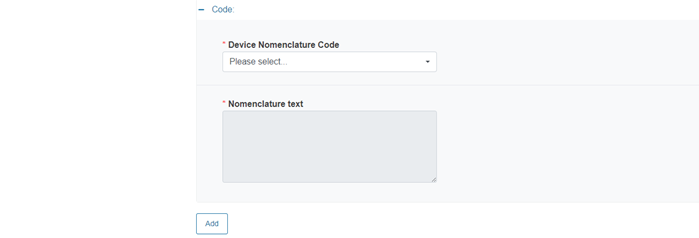
To add more nomenclature codes, click Add.
B. Basic UDI-DI(s)/EUDAMED DI(s):
Enter the Basic UDI-DI/EUDAMED DI:
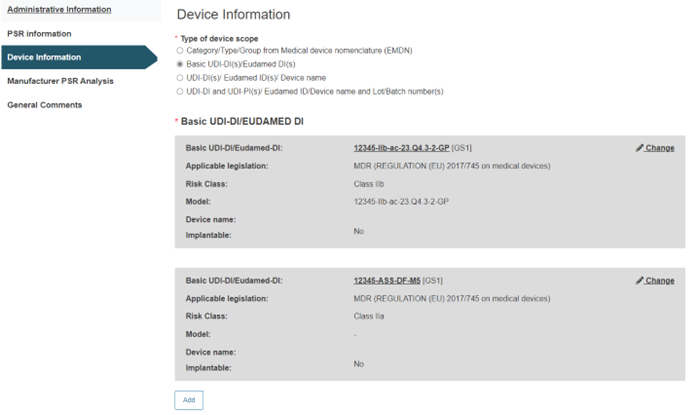
Click on Add to add more Basic UDI-DI(s)/EUDAMED DI(s).
C. Device Identifier(s) /Reference/Catalogue number(s)/Name(s):
Under Device details, click on the plus sign next to Device identifier to select it:
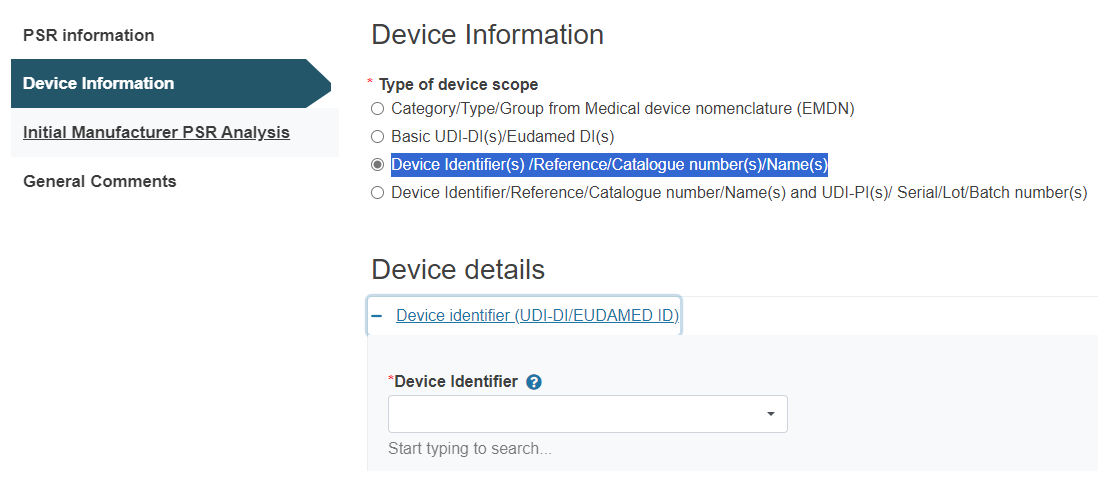
Click Add to add more devices if necessary.
D. Device Identifier/Reference/Catalogue number/Name(s) and UDI-PI(s)/ Serial/Lot/Batch number(s):
Click on the plus sign next to Device identifier to select it:
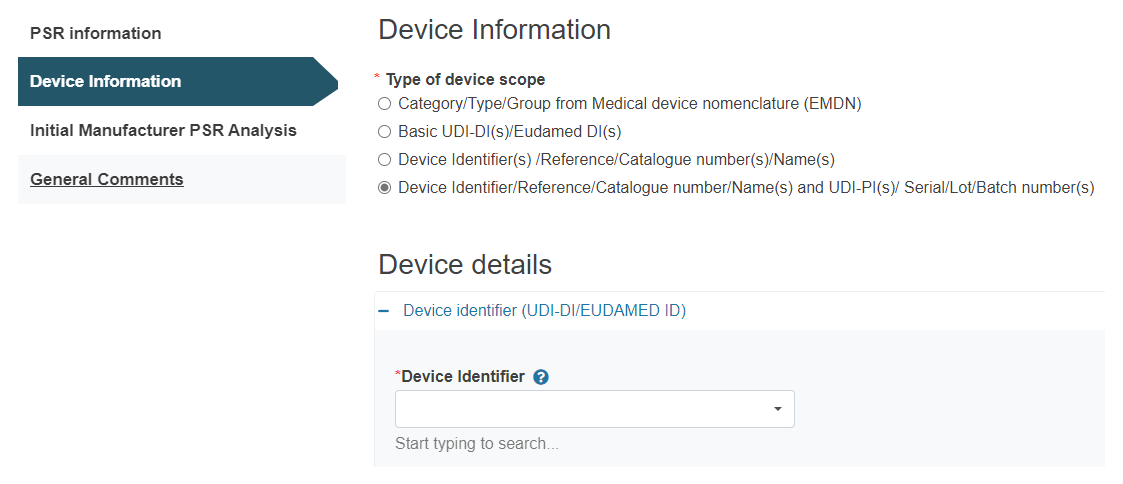
Next, upload the UDI-PI:
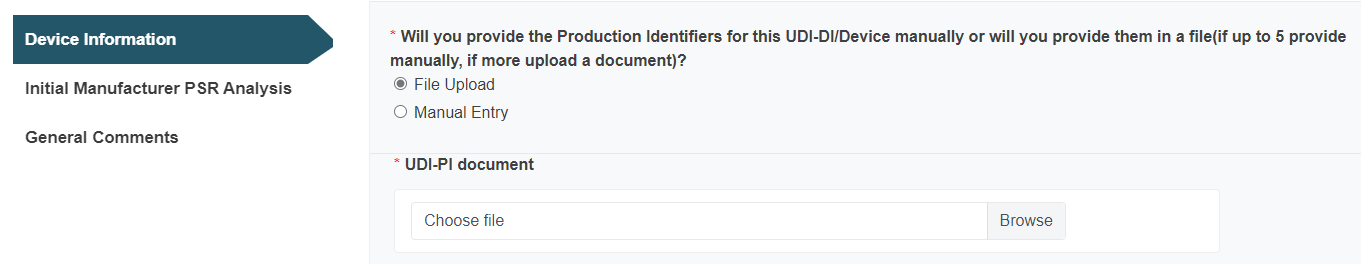
Alternatively, enter the Production Identifier information manually:
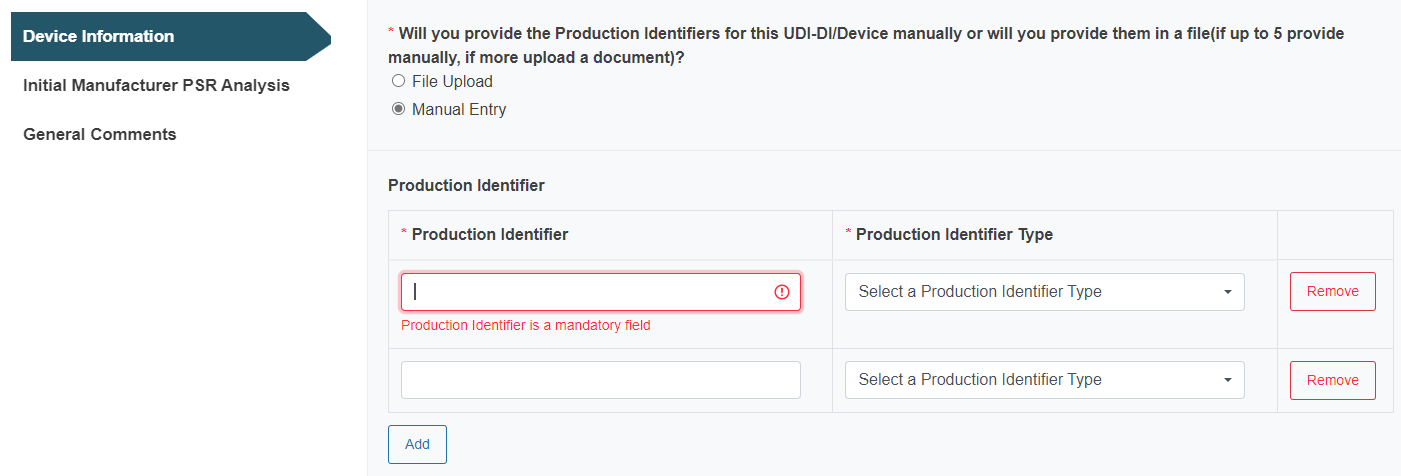
Select the Notified Body ID number and enter the Notified Body certificate number manually:
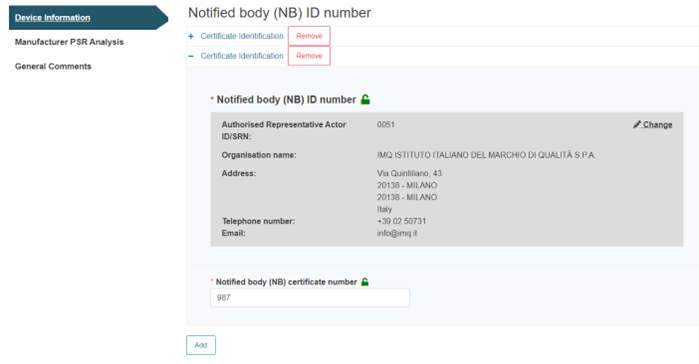
Click Add for multiple entries.