Changing a competent authority for registered actors
The change of responsible Competent Authority (CA) can be necessary in some specific cases as follows:
An EU economic operator that moves within the same country;
A non-EU manufacturer that changes its Authorised Representative or its Authorised Representative changes the responsible Competent Authority;
A non-EU SPP producer that changes the market distribution of their SPPs.
For the non-EU manufacturers, their responsible CA must be at any moment one of the responsible CA(s) of the AR(s) with which they have an active mandate.
On the homepage of EUDAMED, click on Manage your actor data:
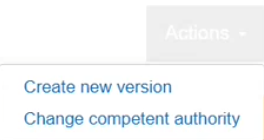
In the new page, click on and then choose Change competent authority:

Note
If a request for Change competent authority has already been submitted, no further editing of the actor data is allowed till the change is either accepted or rejected.
If a draft has already been created, the competent authority cannot be changed.
Note
You will notice the change request in your actor data. The section Competent Authority, will display two parts, Current competent authority and New competent authority:
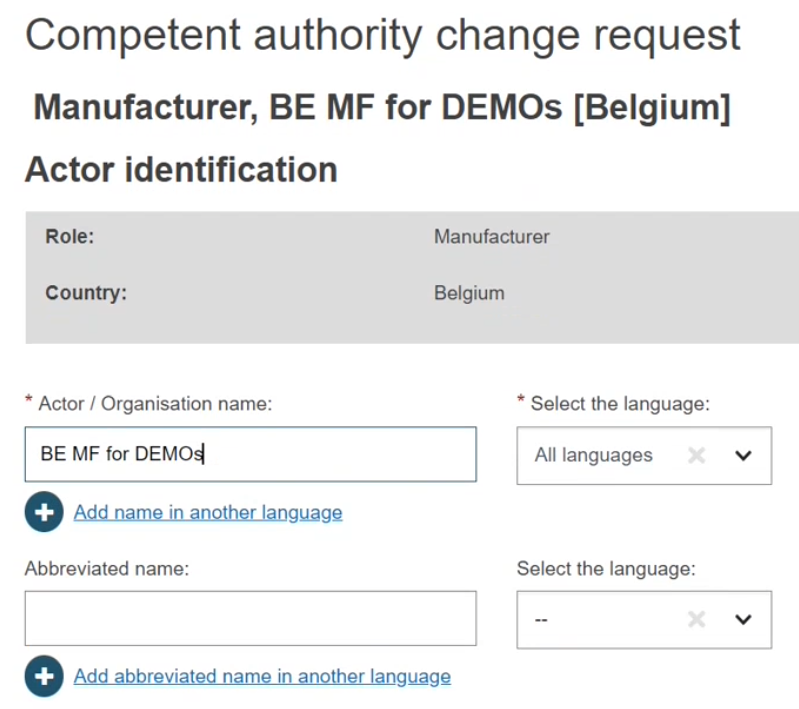
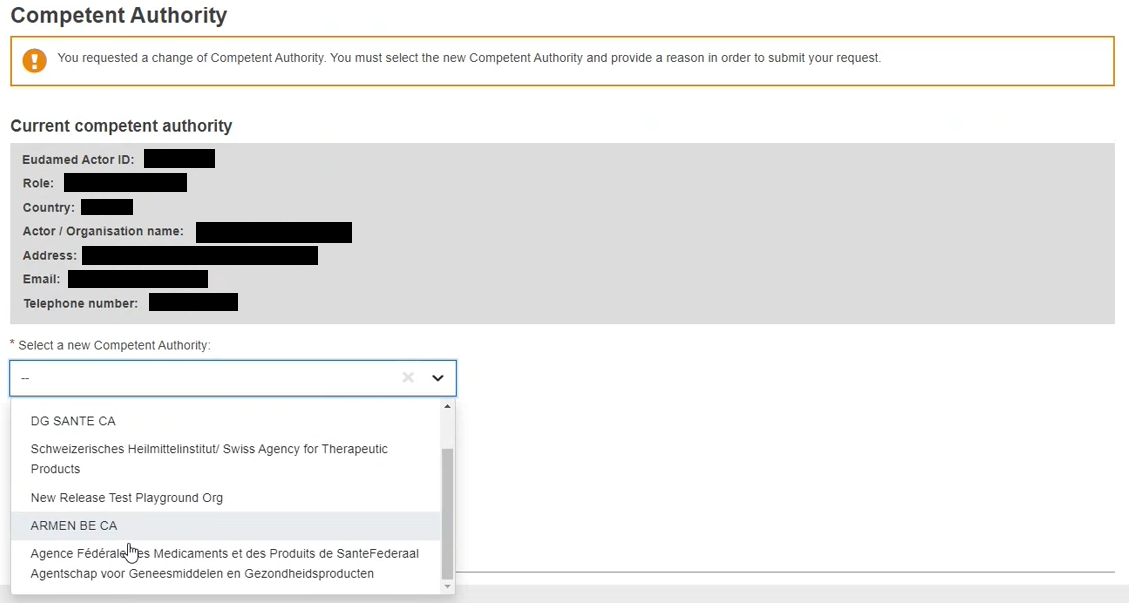
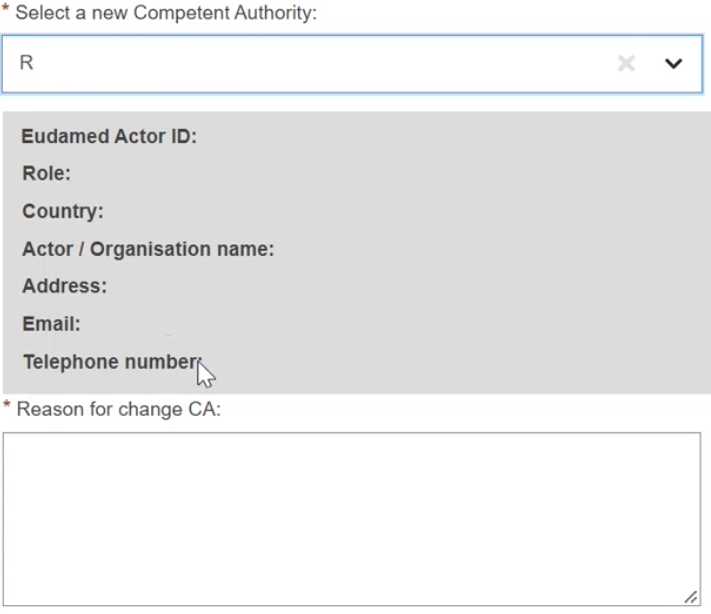
In the new window, you will notice an almost exact copy of your actor data form, with one exception. At the bottom of the page, the section Select a new Competent Authority displays. Choose your new competent authority from the dropdown list:
Note
For non-EU manufacturers, the results in the dropdown list are determined by the responsible Competent Authorities of the Authorised Representative(s) they have an active mandate with.
Once you have selected a new competent authority, enter the reason for the change in the Reason for change CA field:
Click on :

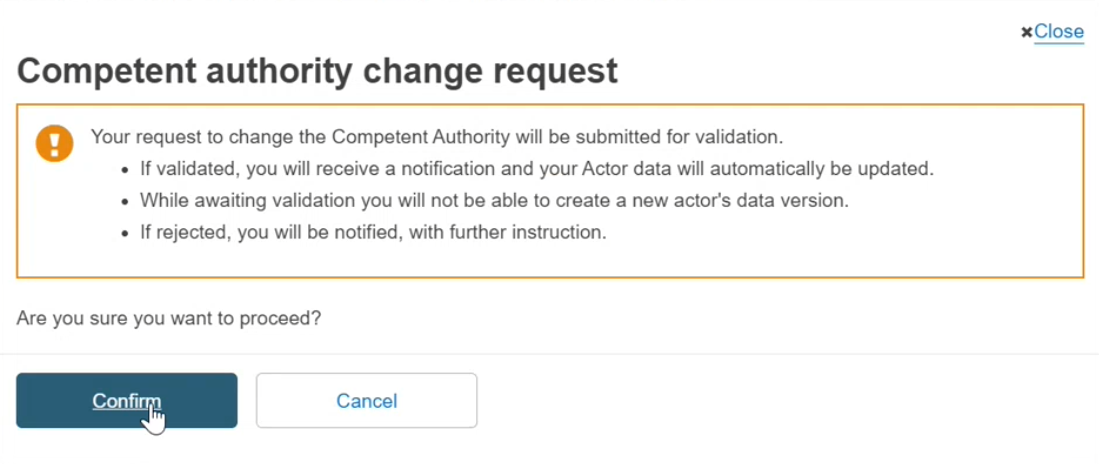
A pop-up message will appear, click on Confirm or Cancel to go back:
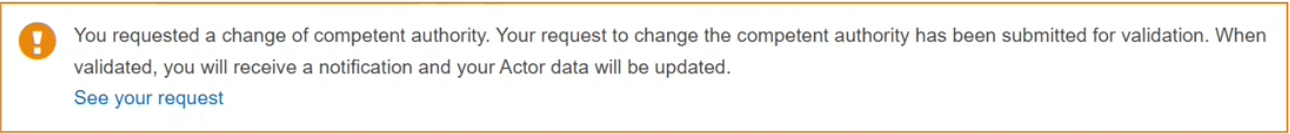
A success message will appear, non-EU manufacturers need to wait for the concerned Authorised Representative to assess the CA change request:
To see your pending/rejected competent authority change requests:
On the homepage of EUDAMED, click on Manage your actor data:
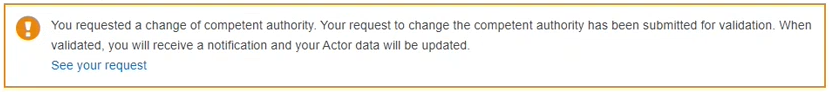
If you have any pending/rejected requests, they will be accessible via the link found in the notice message.
Pending request message:
Rejected request message:

Once you have clicked on the link, you will arrive to an overview of your change request:
Reason for rejection and other details:
