Registration of a UDI-DI for an existing Basic UDI-DI of a Regulation Device
On the EUDAMED Dashboard, select Manage your Basic UDI-DIs/ EUDAMED DIs:
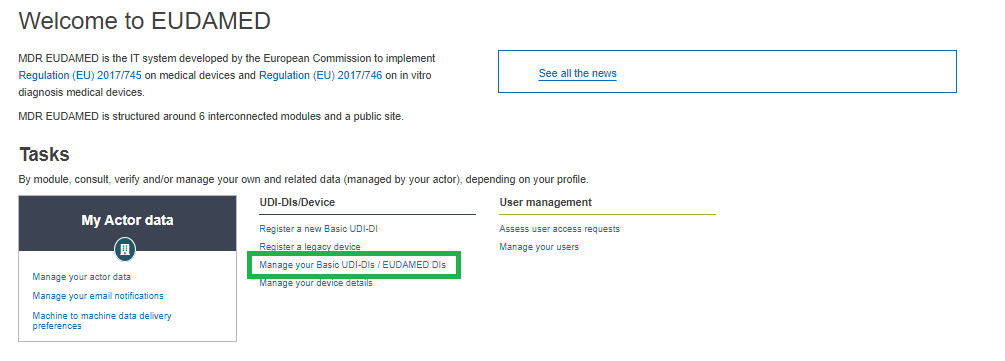
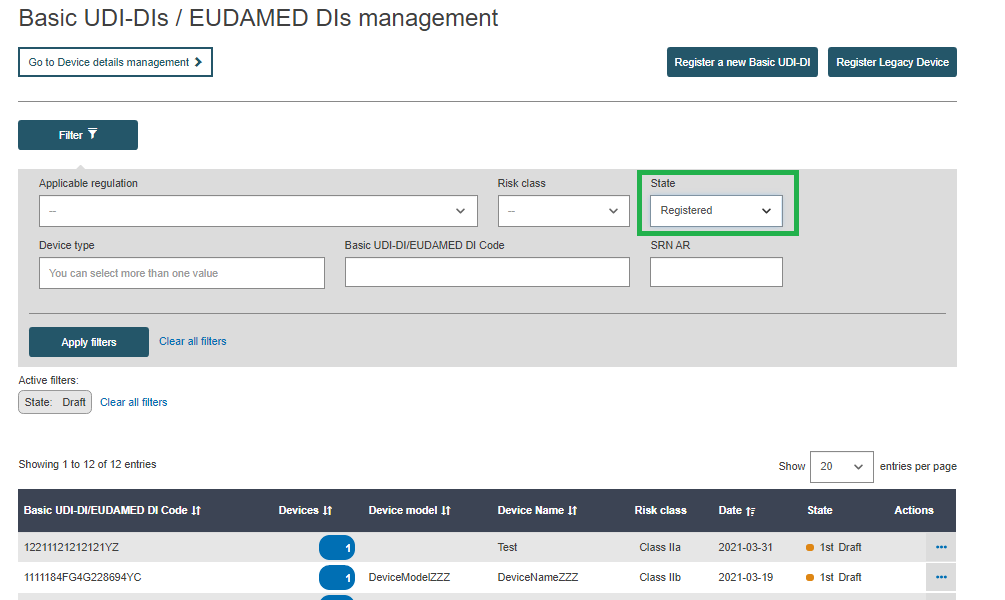
Filter the Basic UDI-DIs/ EUDAMED DIs in state Submitted or Registered:
Important
Additional UDI-DIs for a Basic UDI-DI can be added only for Regulation Devices (not for Legacy Devices).
New UDI-DIs can be added only to Basic UDI-DIs that are in state Registered or Submitted:
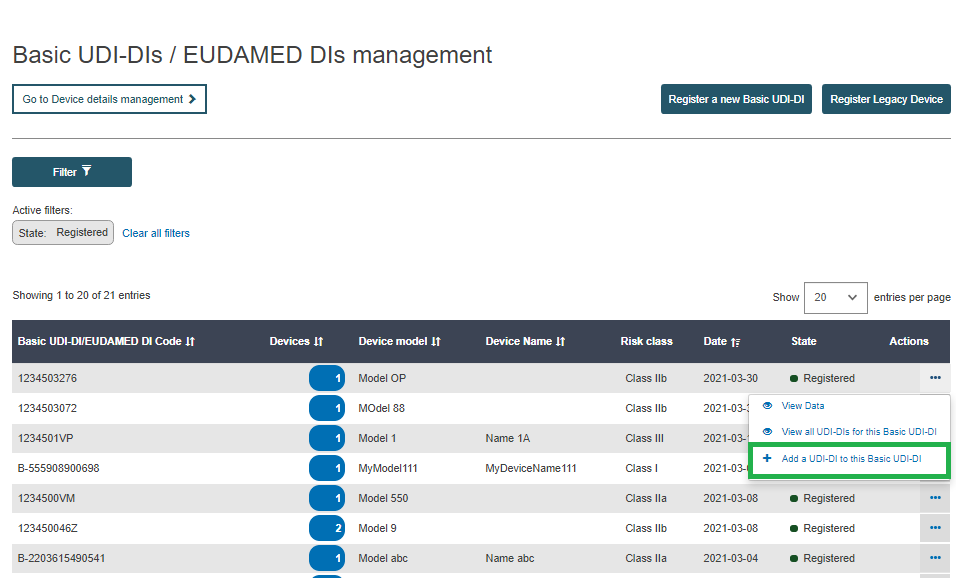
From the results, find the Basic UDI-DI for which you would like to add a new UDI-DI. Click on the three dots on the right and click on Add a new UDI-DI to this Basic UDI-DI:
Complete the series of steps required for the registration of a UDI-DI for an existing Basic UDI-DI ( UDI-DI identification information, UDI-DI Characteristics, Device information, Container Package Details):
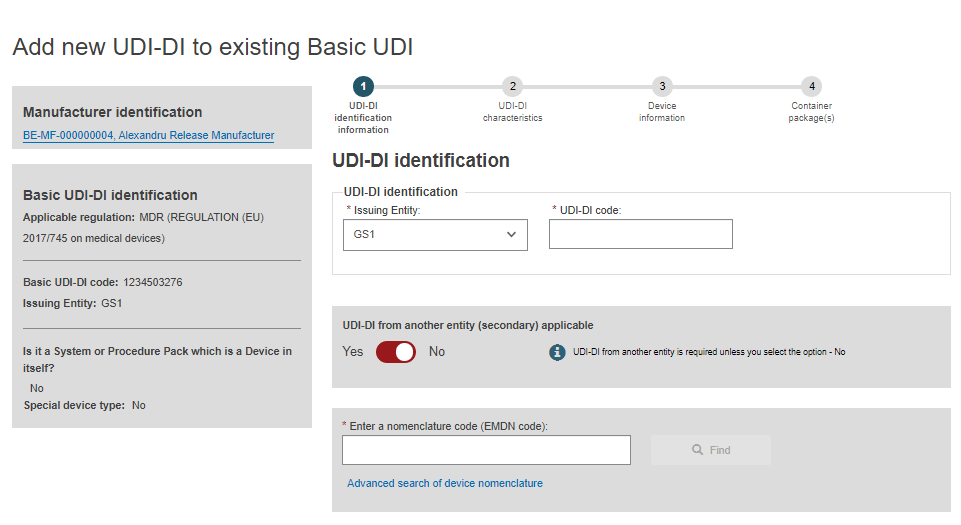
When you have completed all steps, click on Submit my request to submit the new UDI-DI:
Important
After Submitting the UDI-DI, the state of the UDI-DI will be:
Registered if the Basic UDI-DI has the state Registered;
Submitted if the Basic UDI-DI has the state Submitted.