Device information
For MDR, specify whether it is a reprocessed single use device and whether it has an Intended purpose other than medical (Annex XVI):
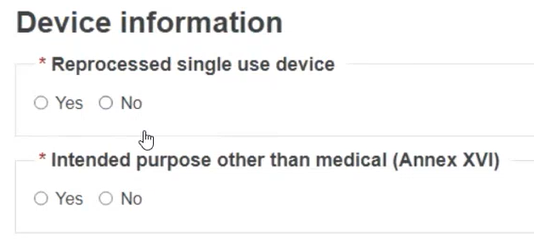
If you select Yes for the Intended purpose other than medical (Annex XVI), possible options will appear. Select the relevant purpose(s):

Select Yes or No if the device was designed and manufactured by another legal or natural person.
If Yes, there are two different ways to find the Product original manufacturer of the device:
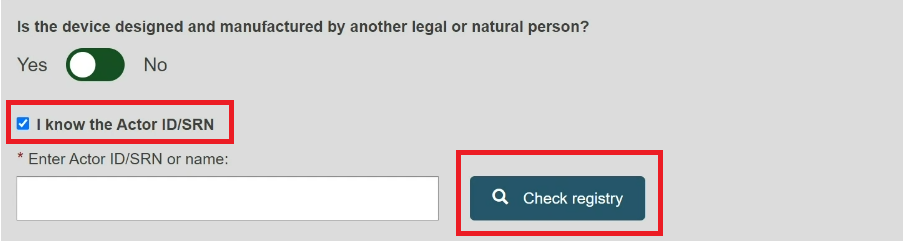
Check the box I know the Actor ID/SRN, enter the Actor ID/SRN or name of the Product original manufacturer of the device and click Check registry:
Note
Please ensure to check the box I know the Actor ID/SRN in order to search for an existing registered Manufacturer Actor either by SRN or by name.
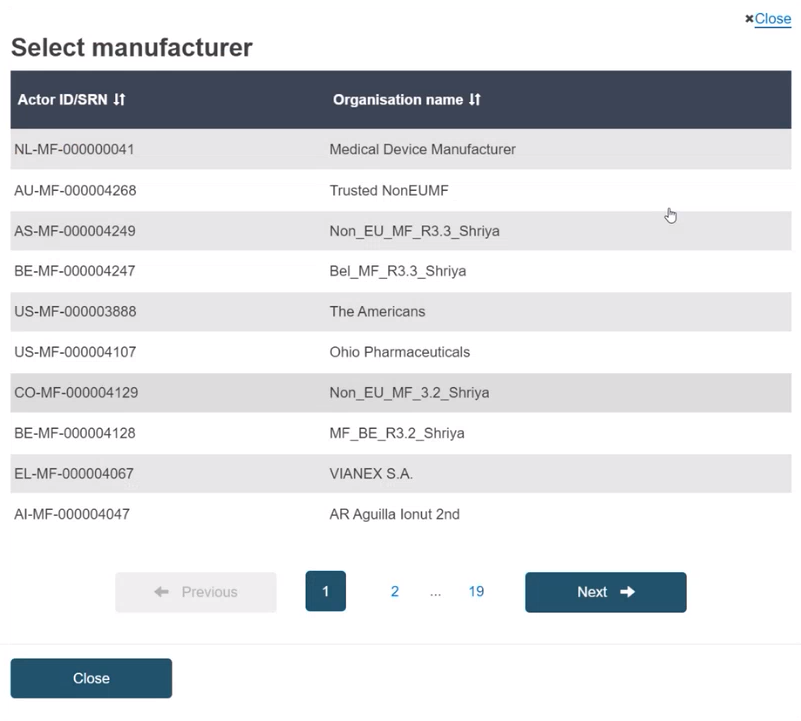
Select the Actor from the list:
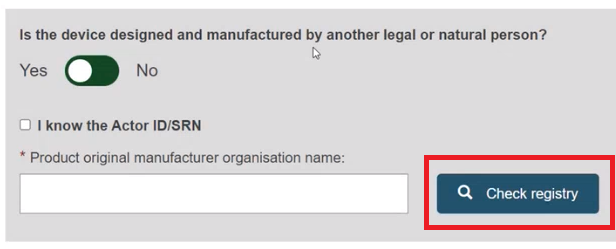
Enter the name of the Product original manufacturer organisation name and click on Check registry:
Select the Organisation name from the list:
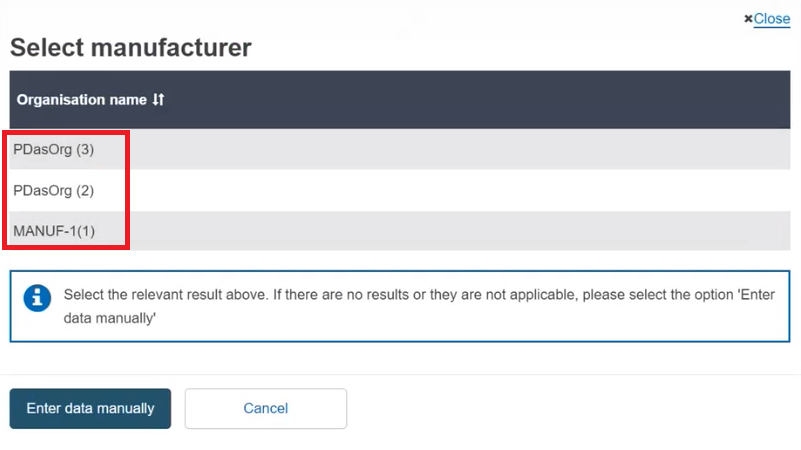
If the Organisation name is not in the list, click on Enter data manually and fill in the required fields with the details on the Product original manufacturer of the device:
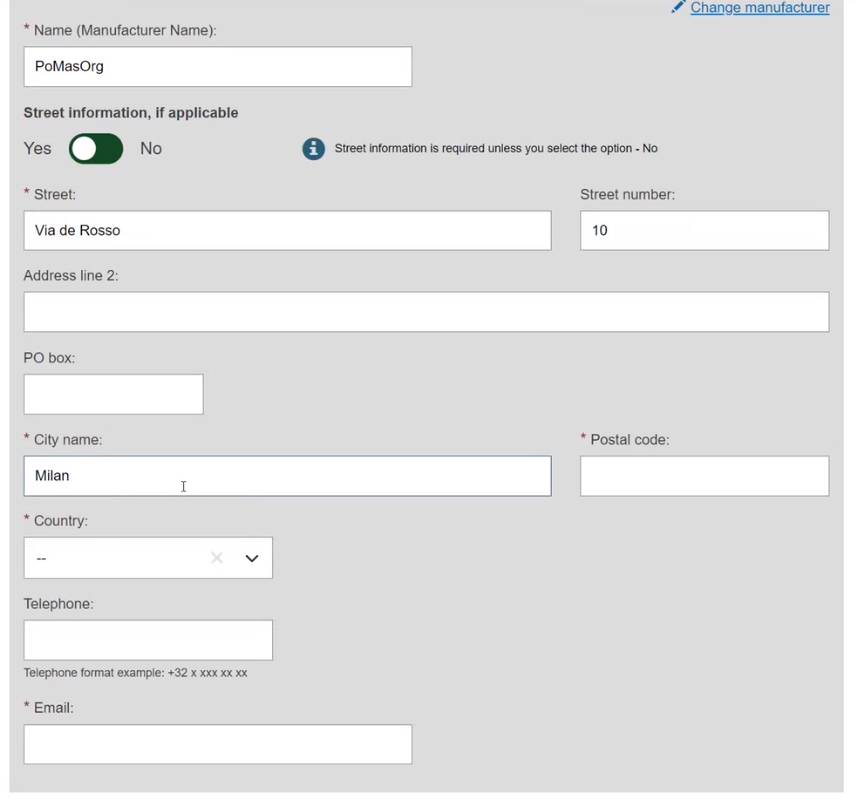
Select Yes or No to provide the Clinical Investigation reference for the current UDI-DI:
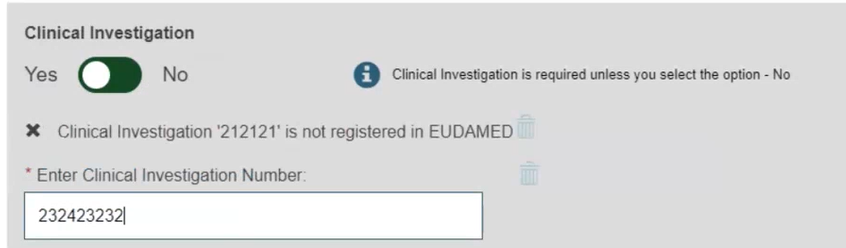
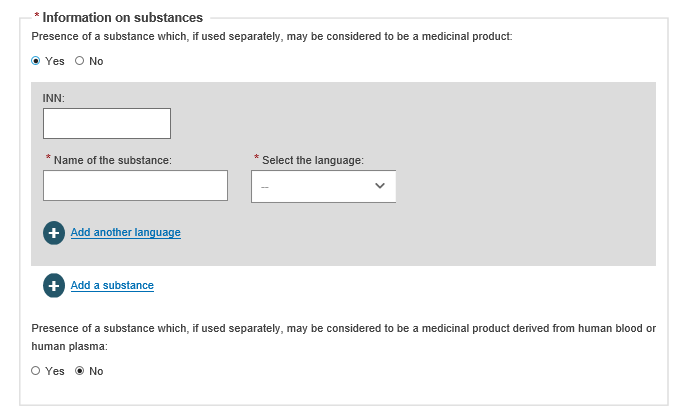
When registering under MDR, select Yes or No to complete information on tissues and cells, and information on substances:
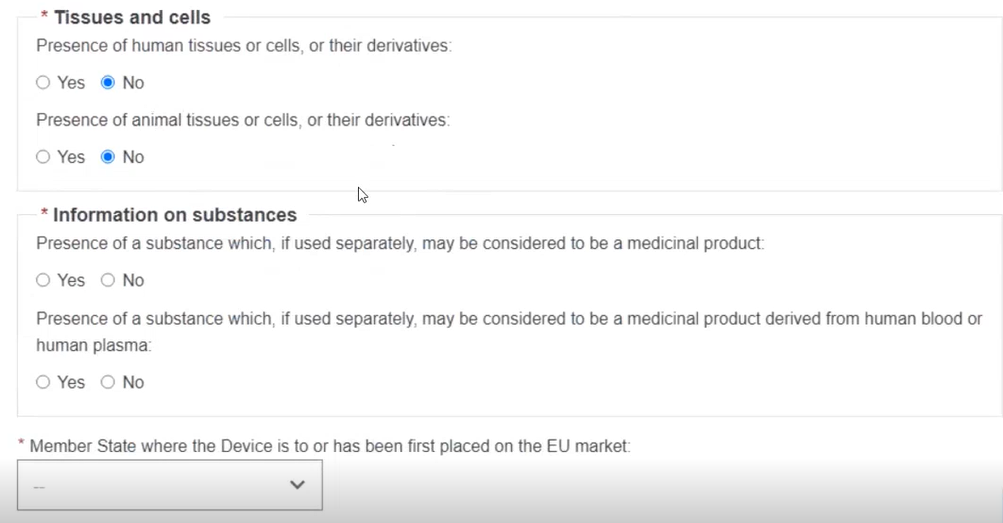
If you answer Yes to Information on substances, enter the details:
For IVDR, select Yes or No to complete information on tissues and cells, in addition you shall specify if the device is new:

Note
A device shall be considered new if:
There has been no such device continuously available on the Union market during the previous three (3) years for the relevant analyte or other parameter.
The procedure involves analytical technology not continuously used in connection with a given analyte or other parameter on the Union market during the previous three (3) years.
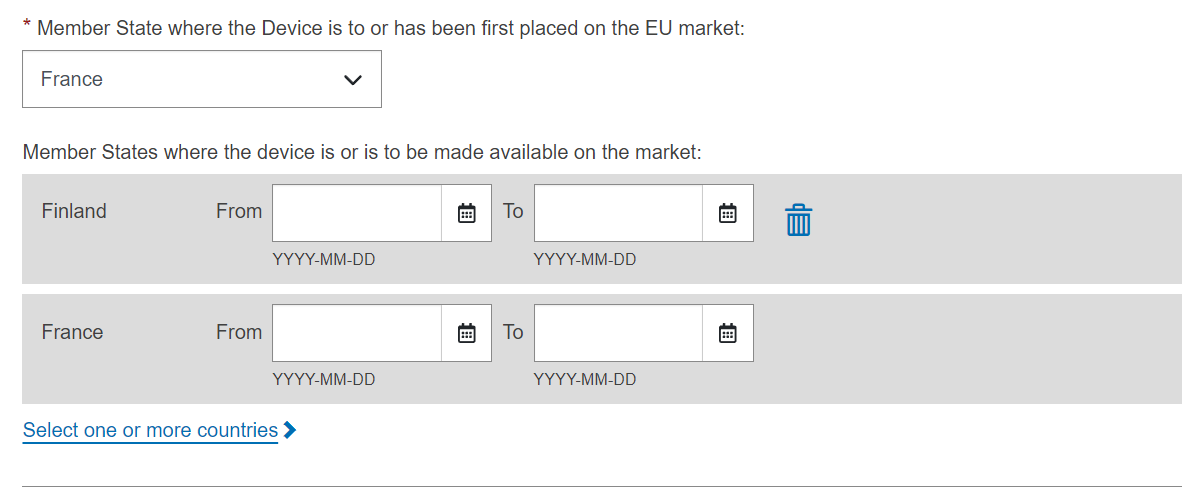
Choose a Member State in the drop-down list where the device is or has been first placed on the EU market, and click Save or Save & Next:
Note
The countries where the device is or is to be made available on the market are mandatory, to be provided when the device’s status is On the EU market and device’s risk class is not risk class I (MDR) and not risk class A (IVDR).