EUDAMED DI identification information
Select the applicable legislation:
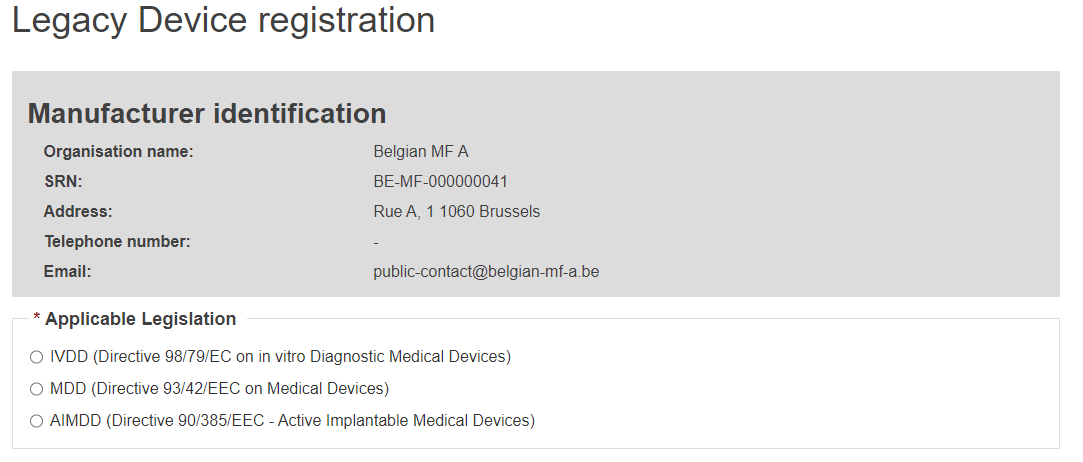
Select Yes or No to whether a UDI-DI is already assigned to the legacy device. If yes, enter the Issuing Entity and the UDI-DI code, and click Generate. EUDAMED will create a corresponding EUDAMED DI (the UDI-DI code with “B-“ as prefix).
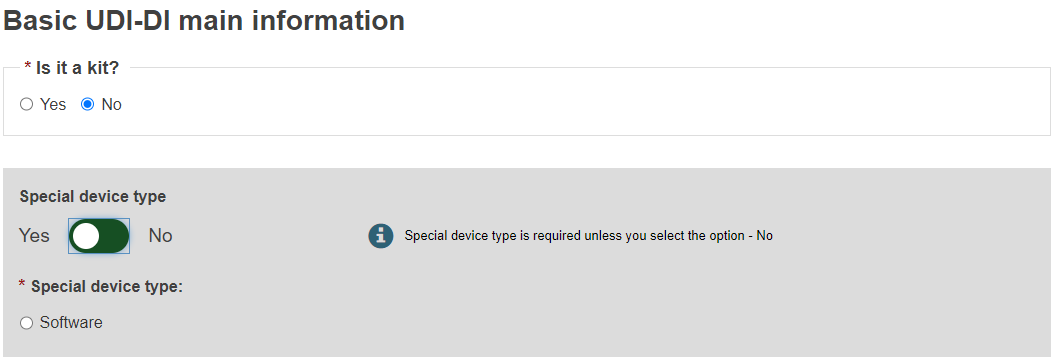
Non-EU manufacturers have to select the authorised representative (AR) for the current device from the options available.
If there is only one AR with an active Mandate with the manufacturer, it will be automatically retrieved:

Click on Save & Next to save it as a draft and continue with the following steps:
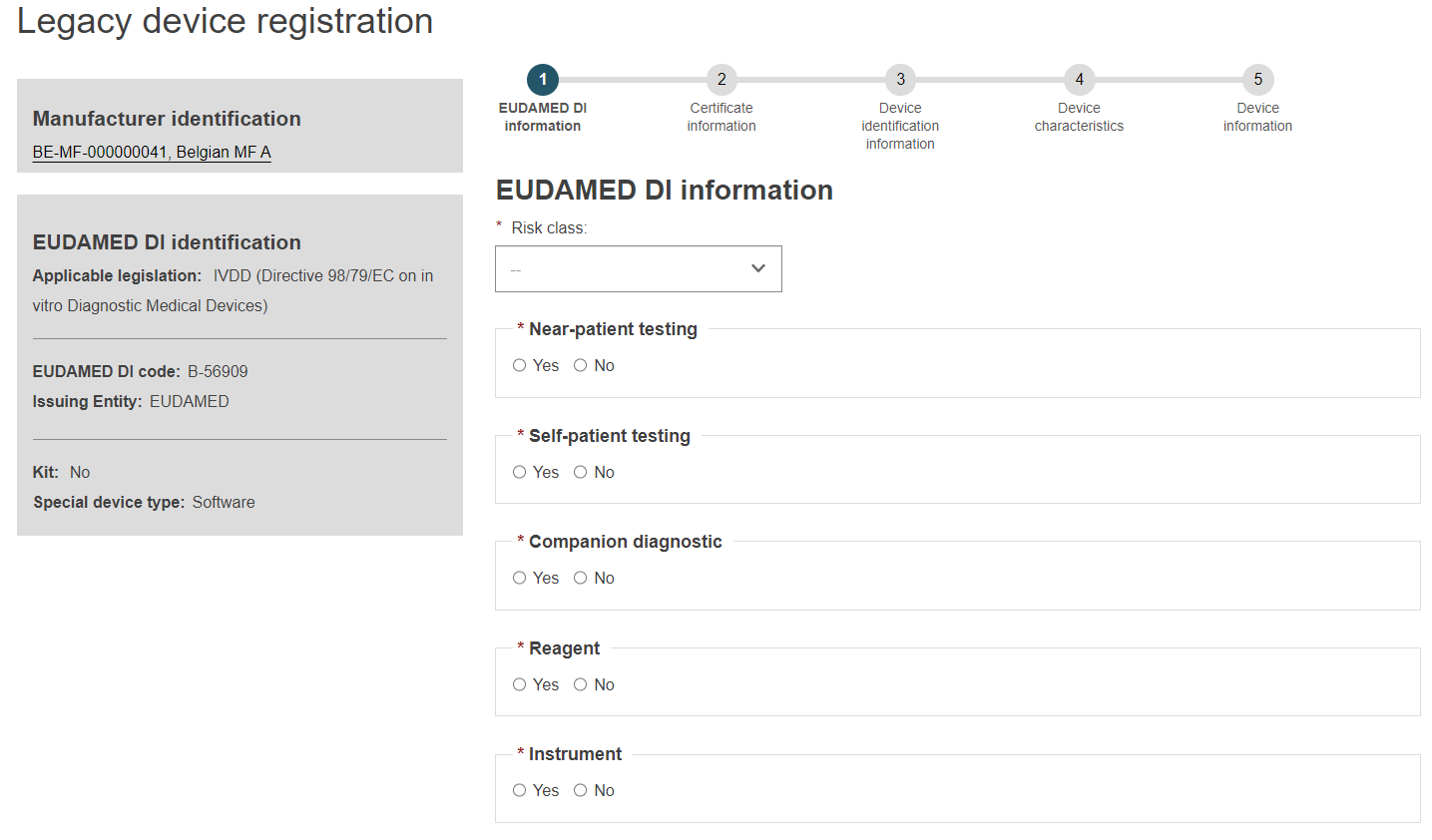
On the left you will see a summary of the device characteristics.
Choose a Risk class from the list and select Yes or No for each of the options.
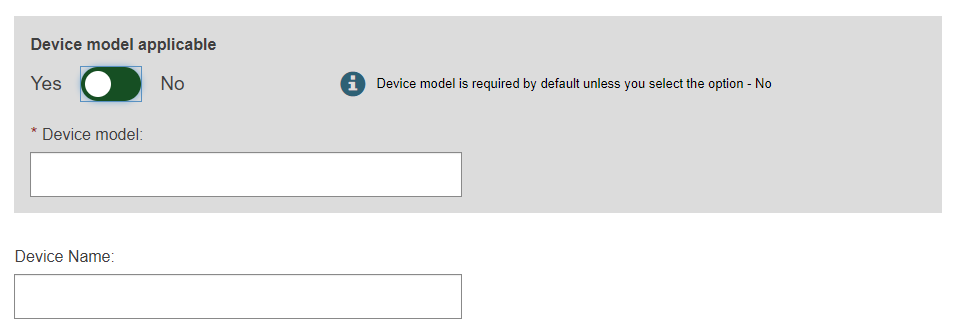
Select Yes or No if the device model is applicable and, if applicable, enter the Device model and enter a Device name if there is one, otherwise enter only a Device name:
Click on Save to save your draft and complete it later, or Save & Next to save it as a draft and continue with the following steps:
