Register new SS(C)P
Click on Manage SS(C)P to navigate to SS(C)P management page from your dashboard:
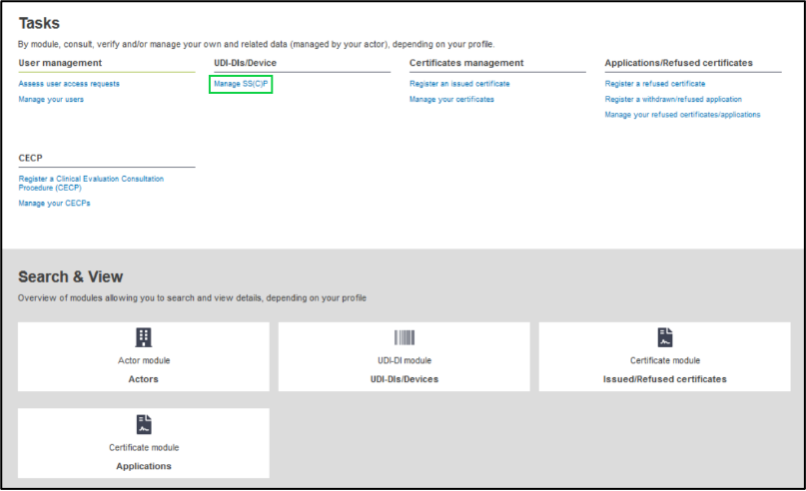
On the next page, click on Create new SS(C)P:
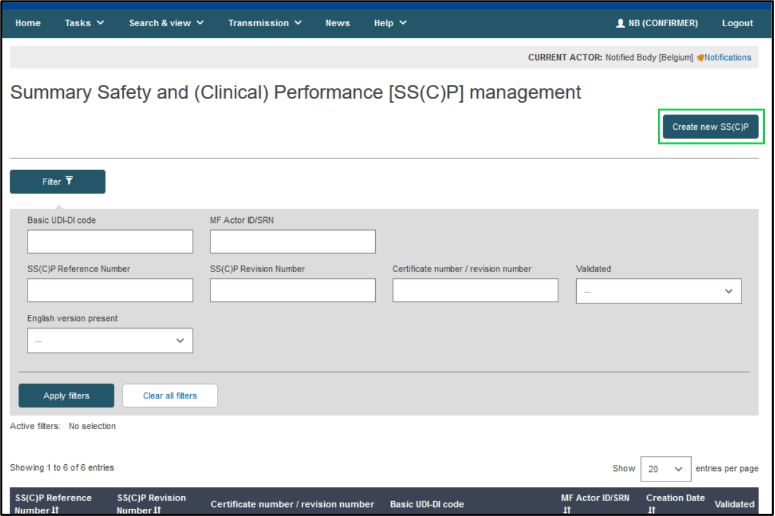
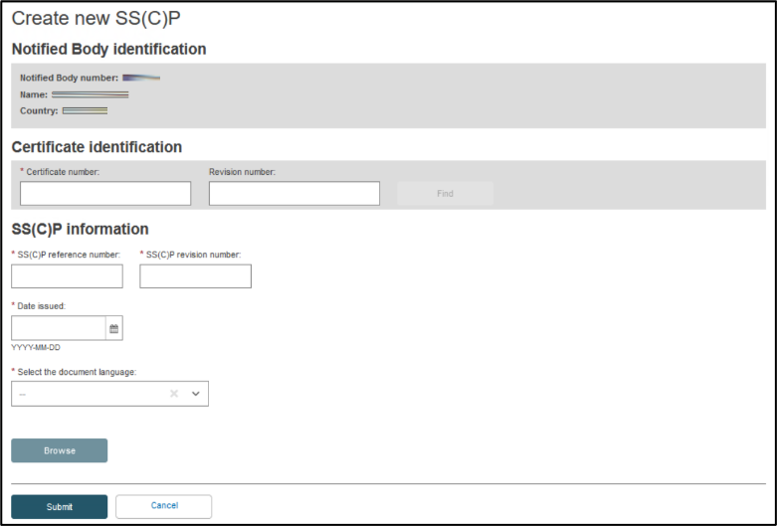
Provide the certificate’s number, and its revision number if applicable, to identify the certificate registered before. Click Find:
Note
Only the following certificate types can be linked to new SS(C)P created within the SS(C)P management page:
– (MDR/IVDR) EU Quality Management System certificate (Annex IX Chapter I)
– (MDR) EU Quality Assurance certificate (Annex XI Part A)
Once the certificate has been identified, its link will be populated in the box along with information about the manufacturer, and, if applicable, the authorised representative:
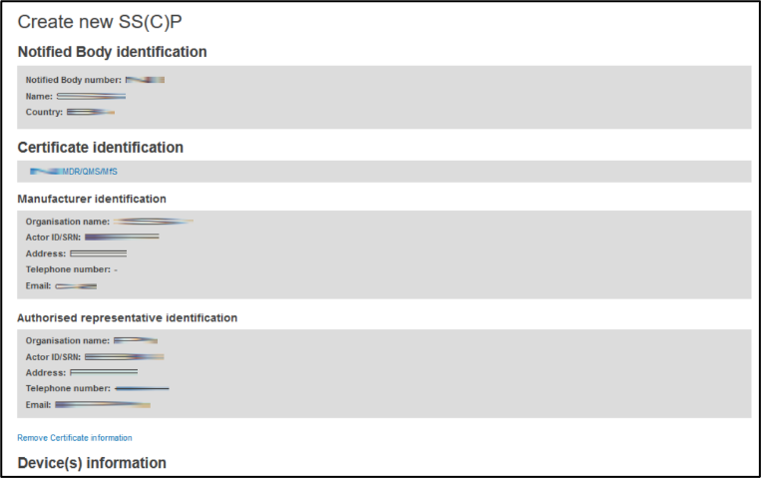
Click the Remove Certificate information link if the certificate displayed is not the intended one. The process to identify the certificate will restart.
Within Device(s) information, enter the Basic UDI-DI to be linked to this SS(C)P:

You may provide at least the first five characters of a Basic UDI-DI and click Search. The system will retrieve Basic UDI-DI(s) according to the Quality certificate types, risk class and their specific characteristics:
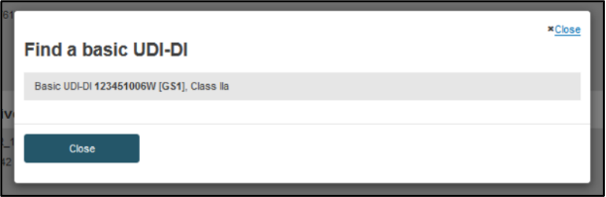
Once a Basic UDI-DI is selected, the system will populate its details:
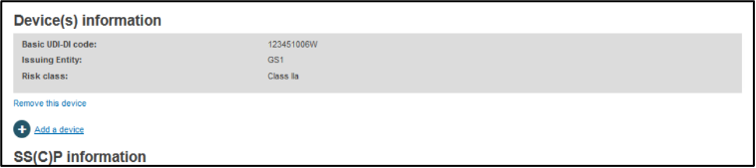
You may add another Basic UDI-DI by clicking on the Add a device link, or remove this Basic UDI-DI by clicking on the Remove this device link.
You need to specify SS(C)P reference and revision numbers, date issued and the language in which the SS(C)P master document is provided. Click Browse to upload the SS(C)P master document. Select Yes if this SS(C)P master document is validated, otherwise select No:
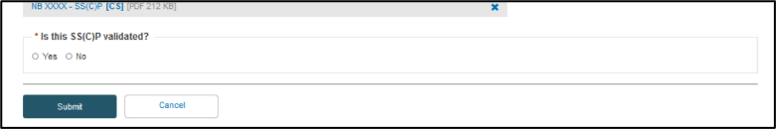
Click Submit and Confirm when asked. A confirmation page will appear:
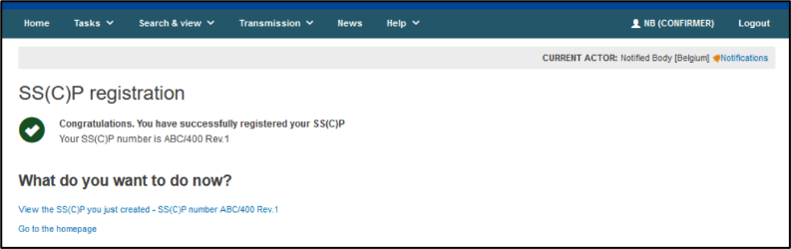
Your new SS(C)P record will appear under the list of SS(C)Ps within the SS(C)P management page.