Merge certificates when re-issuing a Quality certificate
Click on the three dots next to the desired certificate, then click Re-issue Certificate:
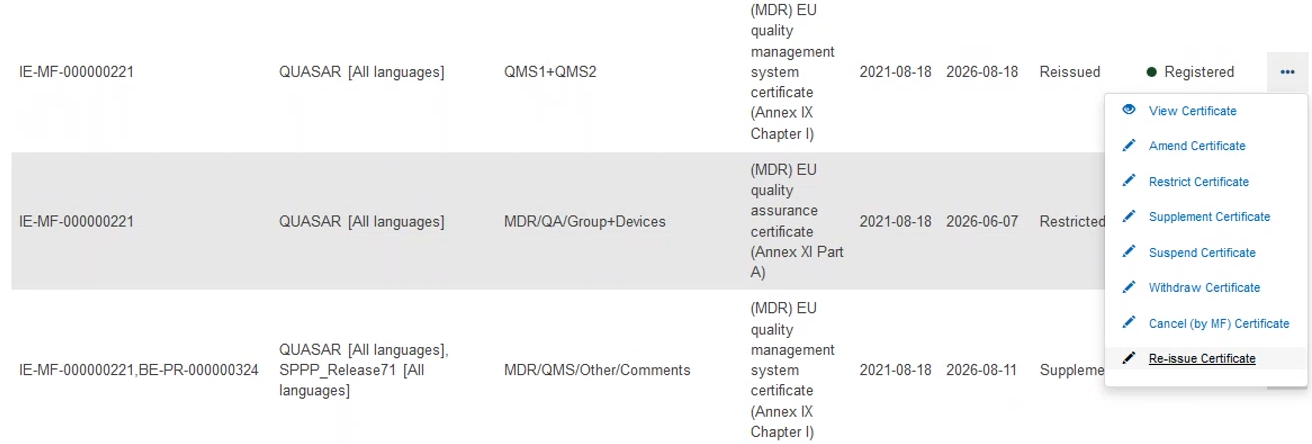
On the next screen, click + Add another preceding certificate:
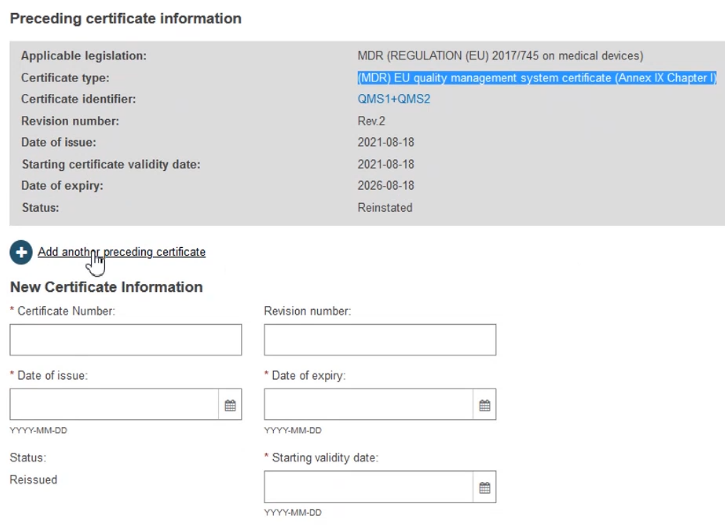
Type the new preceding certificate number and optional revision number then click Find:
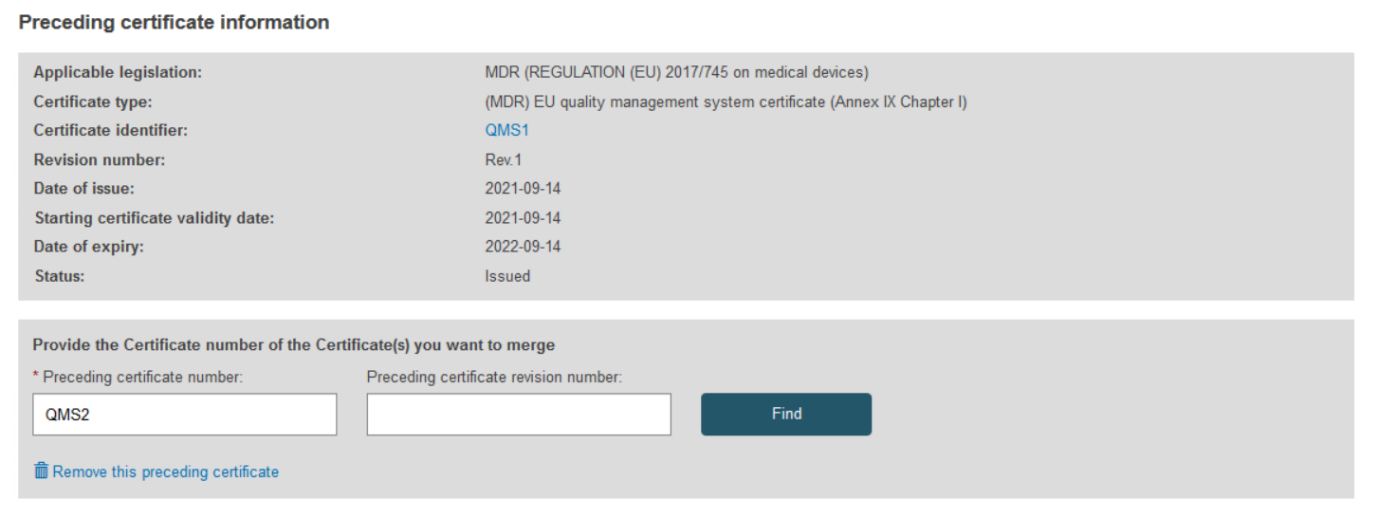
When there is more than one certificate with the same reference number and no revision number is provided, the system will display a selection dialog:
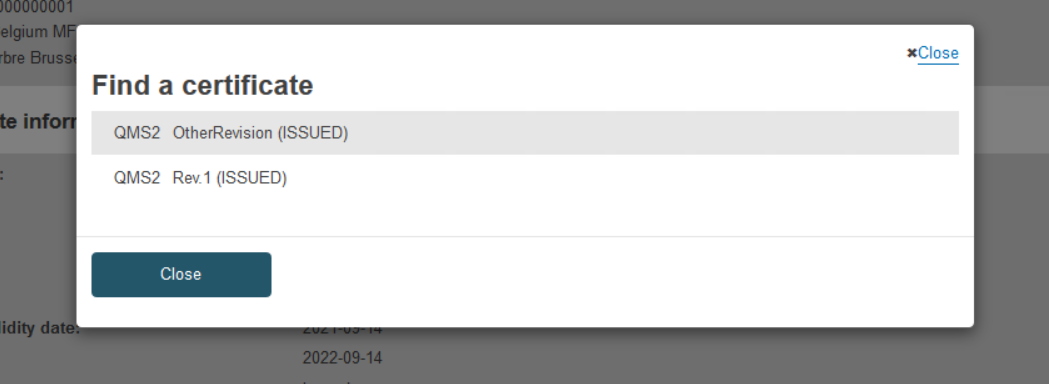
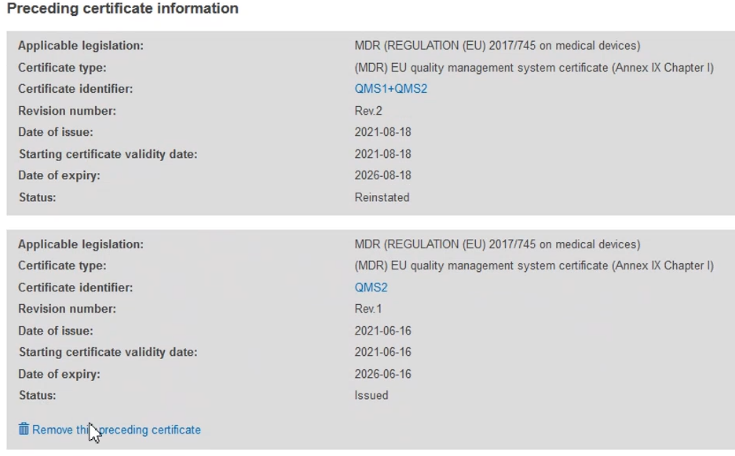
The new preceding certificate information will appear on the list. You have the option of removing it:
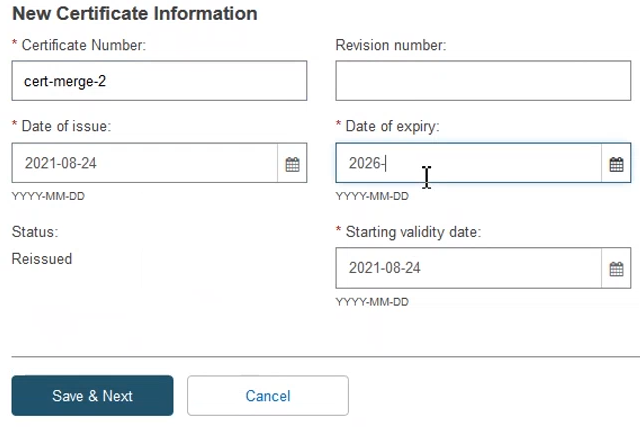
Next, fill in the New Certificate Information and click Save & Next:
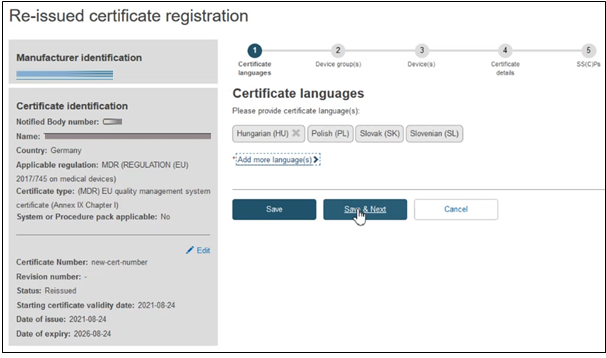
The next screen will display a timeline of steps. Follow the order, starting from the first section Certificate languages.
Click Add more languages if necessary and click Save & Next to complete this step:
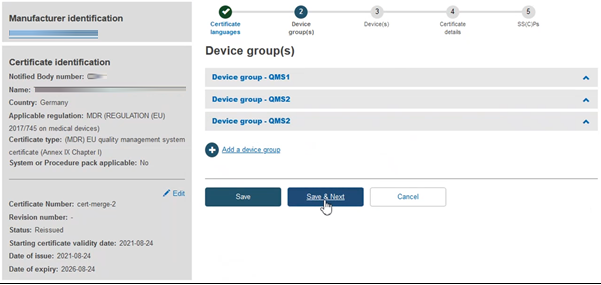
In the next step – Device group(s) – EUDAMED will populate the device groups from the preceding certificate(s), if any. Verify the merged certificate and fill in any required information:
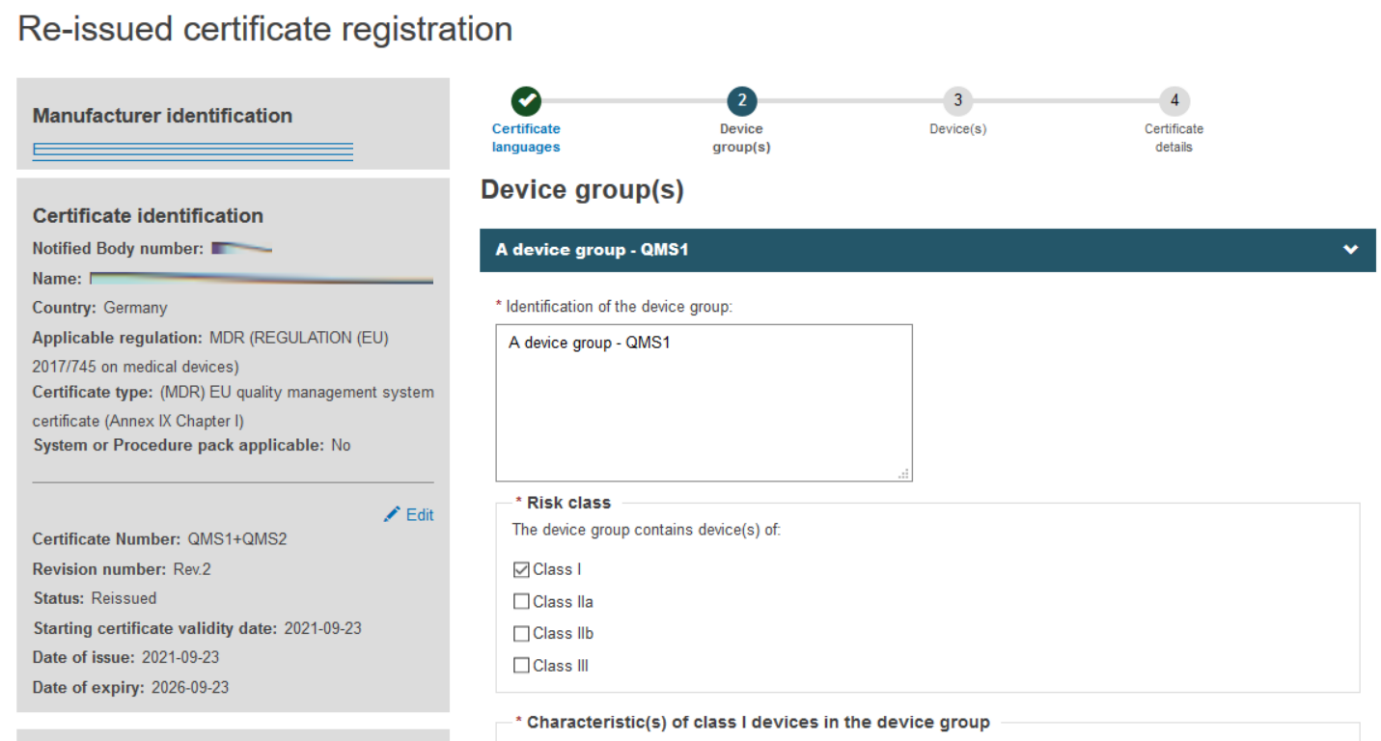
To proceed to the next step, click Save & Next:
As the remaining actions to complete the process are identical to re-issuing a Quality certificate, please consult steps 11-20 of Re-issuing a Quality/Product certificate.