Modules objective
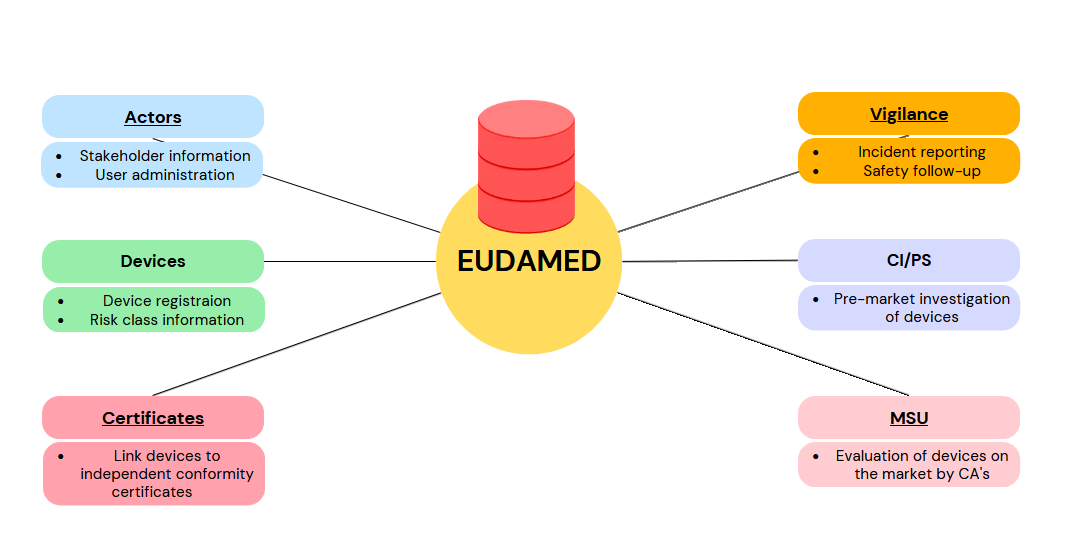
Actors
The Actor module enables Economic Operators to submit, by means of an actor registration request, the information necessary to obtain an actor identifier/single registration number (Actor ID/SRN). The Actor ID/SRN guarantees a EU-wide unique identification for economic operators (also outside of EUDAMED).The module includes the management features for permissions and (access) requests of the organisation's users.
An actor is a natural or legal person (or organisation) with a specific role that has to be registered in EUDAMED.
Actor Data Management:
Name, Address, Contact details etc.
Identification of responsible Competent Authority
User Management:
Granting Access
Changing permissions
Devices
Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) introduce an EU device identification system based on a unique device identifier (UDI) allowing easier traceability of medical devices. This requires that manufacturers submit in EUDAMED the UDI/Device information of all devices they place on the EU market. Manufacturers can already enter UDI/Device information in the system on a voluntary basis.
Devices is a term referring to all peripherals entering the EU that have a medical purpose. For more information click here: Types of medical devices
Certificates
Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) establish that Notified Bodies (NB) should register in EUDAMED any information regarding certificates issued (including amendments and supplements), suspended, reinstated, withdrawn or refused and other restrictions imposed on these certificates. Such information is accessible to the public.
There are two types of certificates: Quality type and Product type.
In this module, there are 2 types of additional information related to devices in EUDAMED:
SS(C)P – Summary of safety and (clinical) performance
CECP – Clinical Evaluation Consultation Procedure
VIG – Vigilance and Post-Surveillance
Medical device manufacturers selling in Europe are legally obligated to report adverse events, incidents, and recalls, also known as vigilance reporting.
CI/PS – Clinical Investigation and Performance Study
Before a new medical device can be put on the market, a Clinical investigation and Performance study for said device needs to take place.
The Clinical investigation/Performance study (CI/PS) module encompasses:
The submission and assessment of CI/PS applications
The submission and potential acknowledgement of Post-market clinical follow up (PMCF) and Post-market performance follow up (PMPF) notifications
Their (substantial) modifications.
It follows the lifecycle of a CI/PS or PMCF/PMPF performed by the sponsor, including any Serious adverse event or device deficiency (SAE/DD) that occurs during the execution of the CI/PS and including any Corrective measures taken by the responsible Competent Authority.
MSU – Market Surveillance
The Market Surveillance module serves to register the activities and the outcome of those activities performed by the Competent Authorities (CAs) in order to survey the market. The information of the module is communicated to all CAs and where relevant the Notified Bodies. Some information is available to the public.