Provision of SS(C)P documents
For implantable devices of class IIa, some implantable devices of class IIb, and devices of class C that are not companion diagnostic and not self/near-near-patient testing, the provision of a Summary of Safety and Clinical Performance SS(C)P is required. [4]
 INFOGRAPHIC: SS(C)P steps and principles
INFOGRAPHIC: SS(C)P steps and principles
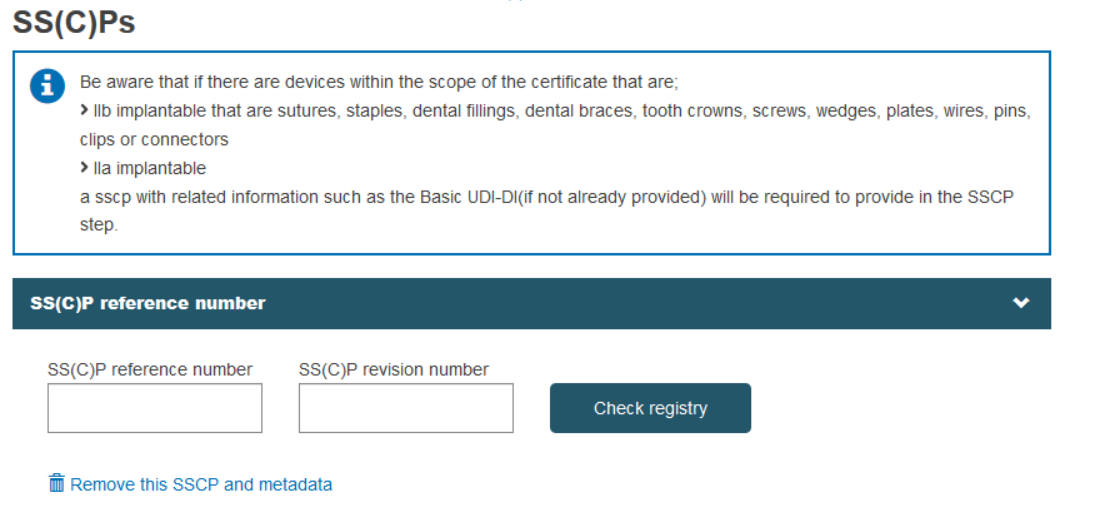
An SS(C)P can be attached to certificates, so the initial dialog requires the provision of SS(C)P reference number and SS(C)P revision number. Once provided, click Check registry. If the SS(C)P exists already in EUDAMED it will be selected and displayed. Otherwise, you may enter a new SS(C)P. You may also create a new SS(C)P version of an already registered SS(C)P (see Create a new SS(C)P version of a registered SS(C)P).
Enter the issued date and select the language of the document. Click Browse to upload the SS(C)P master document:
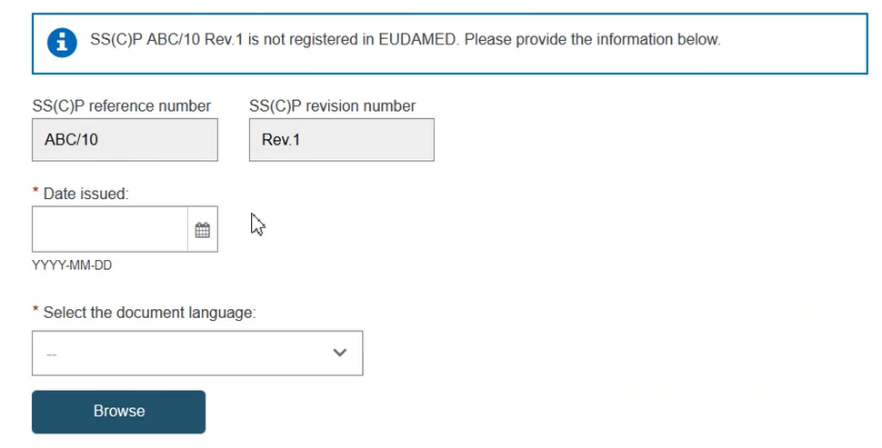
Click Yes to the question Is this SS(C)P validated to indicate that the uploaded master document is validated, otherwise click on No:

In order to register an SS(C)P, the reference of the related Basic UDI-DI(s) within the scope of the certificate is required. Within the Device(s) information box, a respective Basic UDI-DI can be added:
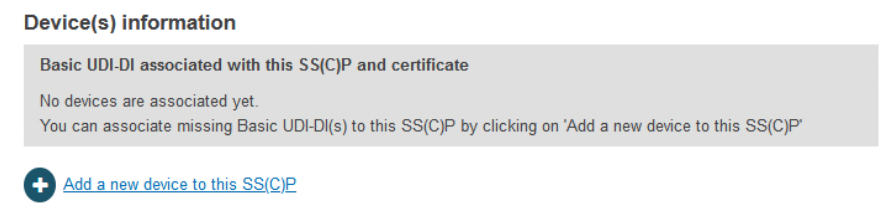
The Device(s) information box confirms no associated devices. Click Add a new device to this SC(C)P. The pop-up shows two sections: the Basic UDI-DI(s) referenced in this certificate of class IIa implantable and relevant class IIb implantable or relevant class C, and (for Quality certificates only) a list of all Basic UDI-DIs of class IIa implantable and relevant class IIb implantable or relevant class C registered by the referenced manufacturer. You can select multiple devices:
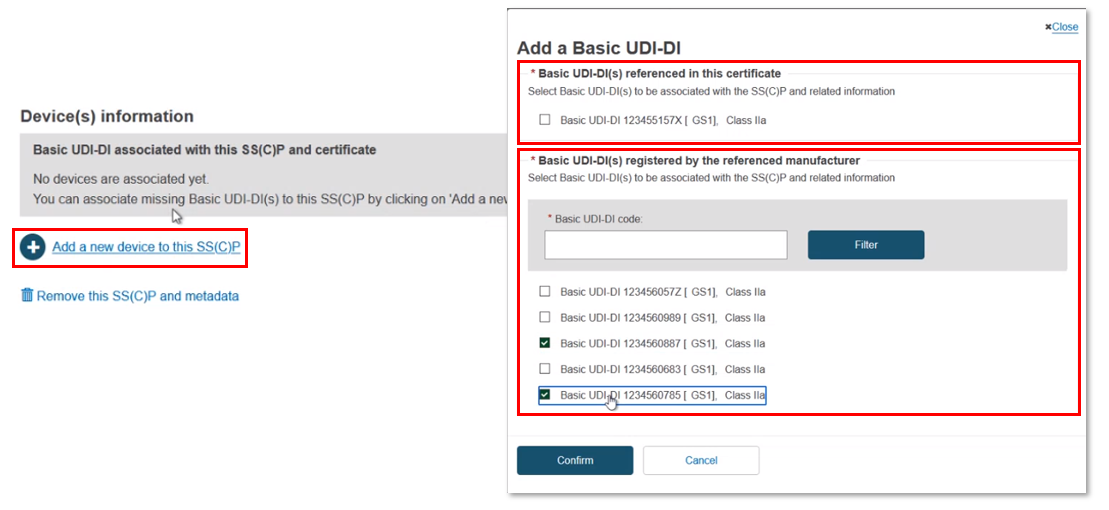
You can input a partial Basic UDI-DI number and click Filter to narrow down the search results:
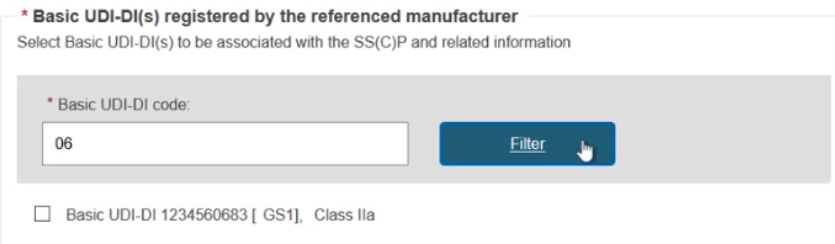
Once selected, click Confirm to link the devices to this new SC(C)P. Nothing is yet submitted, and you can delete this SS(C)P by clicking Remove this SS(C)P and metadata to return to the previous screen.
When finished, click on the Preview button to review the provided information:
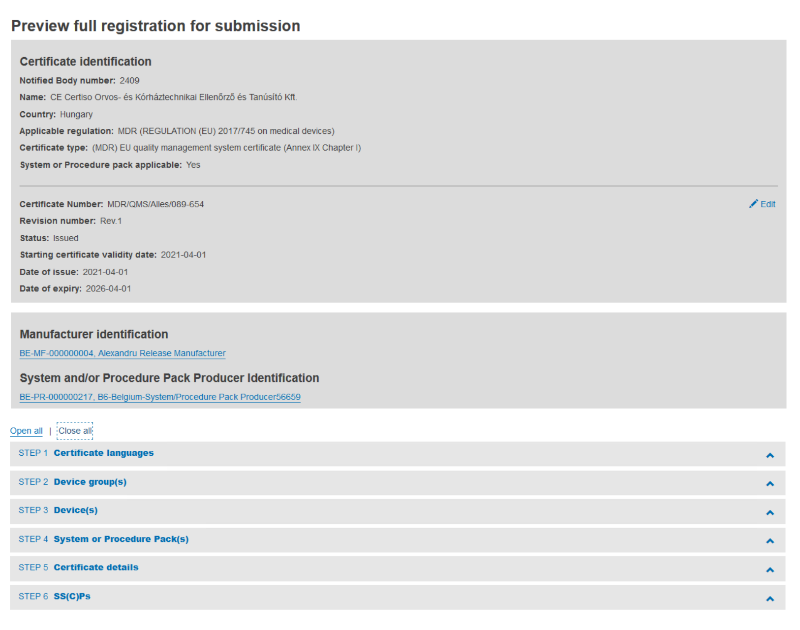
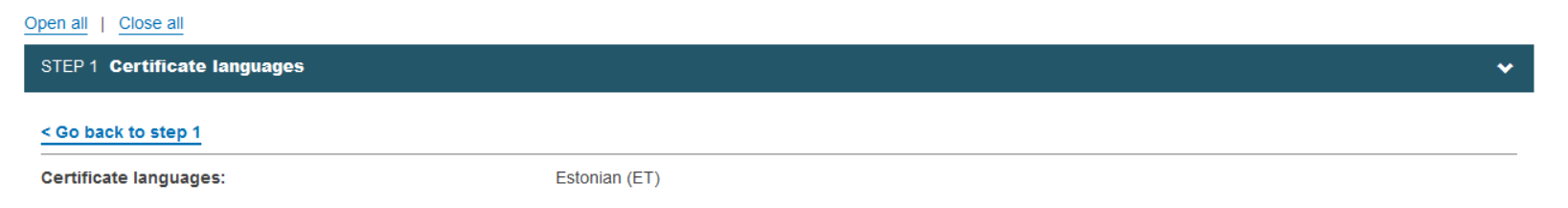
From this page you can easily access other steps by clicking the respective link:
When you click Submit and confirm your submission, the certificate will be registered in EUDAMED and you will see a Confirmation page:
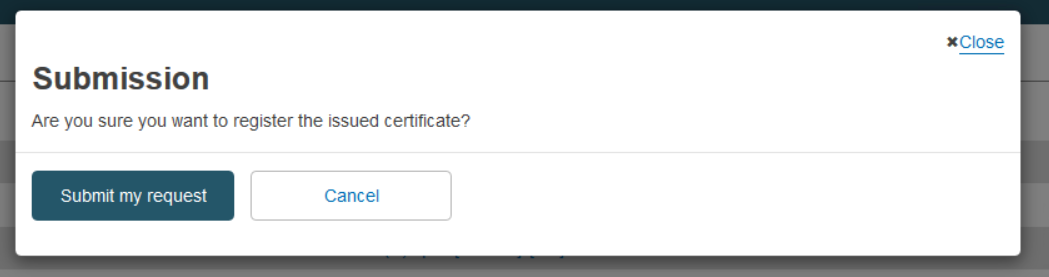
From the confirmation page you can click on View the certificate you just created to open the registered certificate view page, or you can click on Go to homepage to return to your homepage.
[4] For background information, see the MDCG guidance: Summary of safety and clinical performance A guide for manufacturers and notified bodies.
