Certificate information (when applicable)
This section will become active depending on the information provided for Risk Class and additional properties in the Basic UDI-DI.
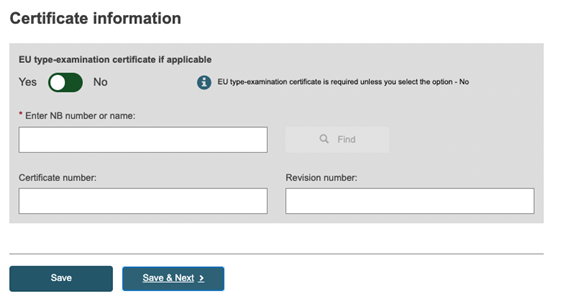
In the case of certificate information, at least the following should be provided:
whether EU type examination certificate is applicable.
the Notified Body (NB) responsible for the product certificate.
if known, the certificate identification.
Additionally, more information on the certificate type could be required depending on the risk class and properties specified for the Basic UDI-DI. For the NB, enter some or all of the NB name or number, click Find and choose the correct Notified Body from the new window.
If known, enter the certificate number and revision number and click on Save or Save & Next.
Note
Certificate Information for a Basic UDI-DI registration is applicable only when its confirmation by the Notified Body from the certificate registration is required (as specified in Art 29(3) MDR/Art 26(2) IVDR).
In Annex 1 – Device Certificate Information you can find the different cases in which Certificate information is needed and the type of certificate. (In summary, it is applicable for MDR risk class III and IIb and IVDR risk class B with self-patient testing/near-patient testing, risk class D and C).
 |