PSUR details / Submission
Click on the PSUR details tab from the menu on the left:
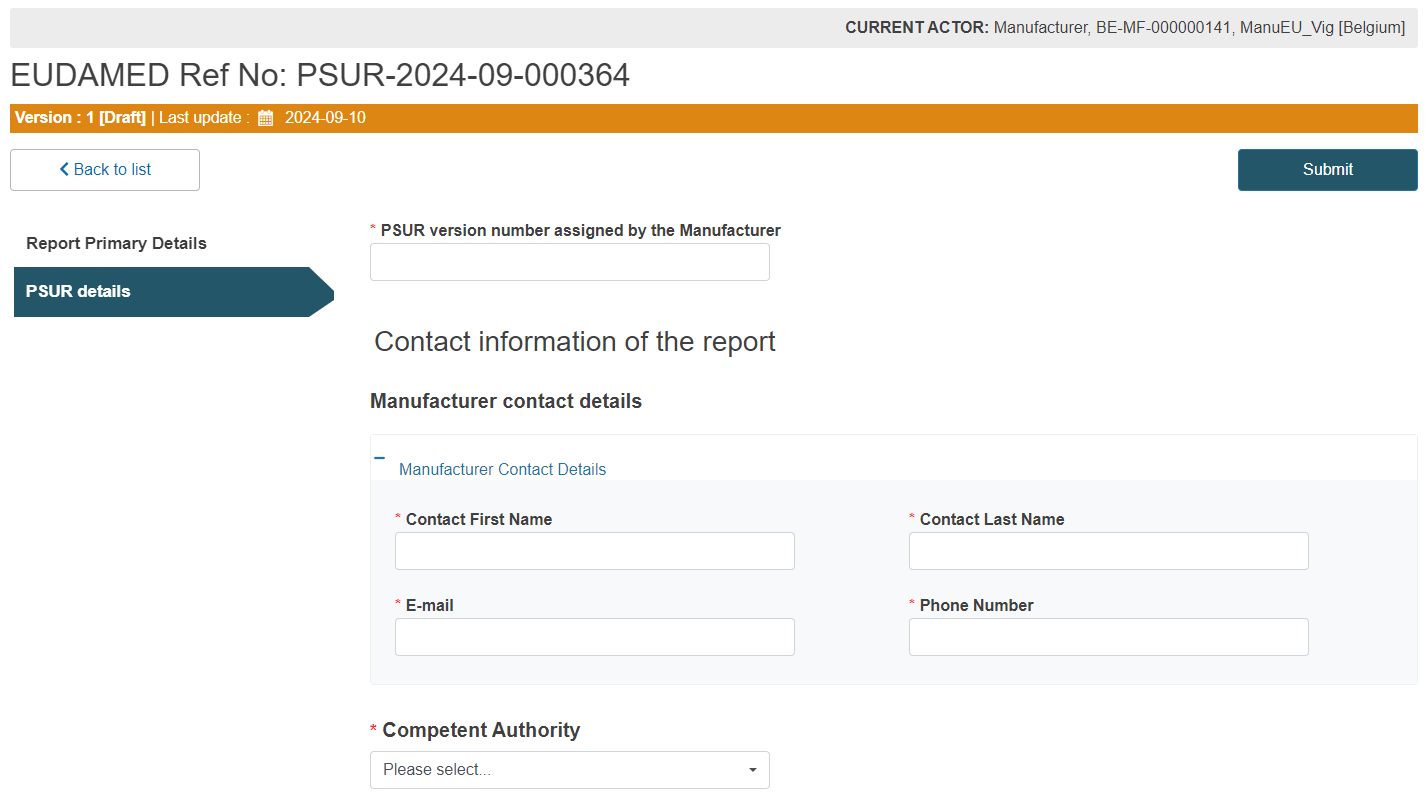
Enter the PSUR version number assigned by the Manufacturer and fill in the Manufacturer contact details:

Select the Competent Authority in the manufacturer’s place of business from the dropdown (this CA will receive notifications regarding this PSUR):

Select the Notified Body which will provide an evaluation for this report from the dropdown:

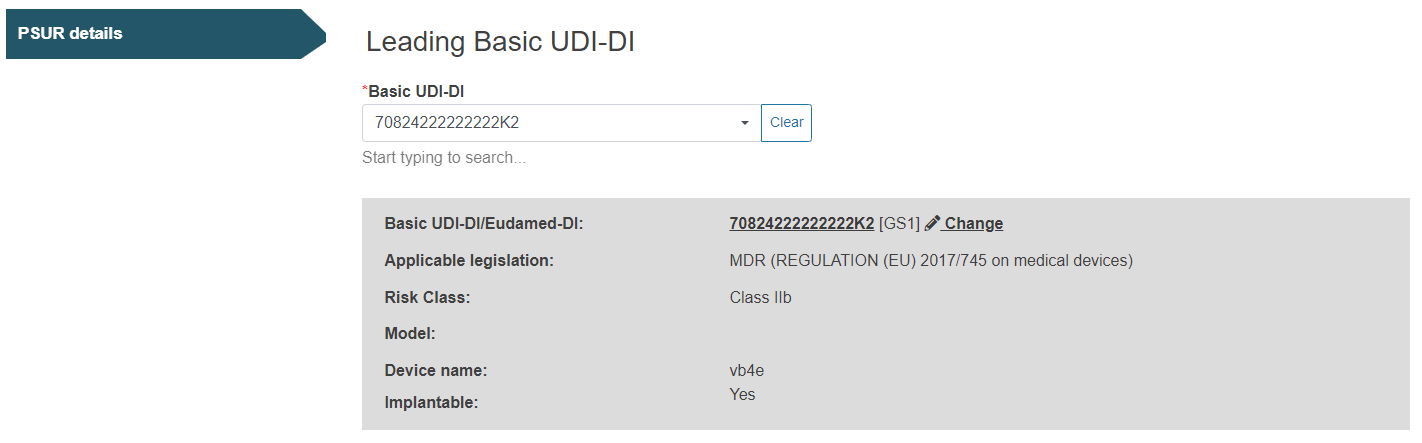
Select the Basic UDI-DI for the Leading Basic UDI-DI for the current PSUR:
Click on the plus sign next to Certificate Identification to access the fields for completion:

Fill in the Notified Body details and the NB certificate number in the text box below:

Tip
If the device has a certificate linked to it, the user will be able to select the certificate number from the drop-down and the system will auto-display the NB ID number. Otherwise the user may fill in the info manually.
If other devices apply to the current PSUR, fill in the Additional Devices section:

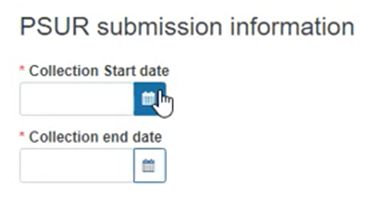
Fill in the PSUR collection dates:
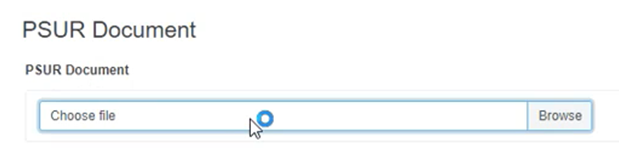
Click on Browse to upload the PSUR document in PDF format:
Submission
Click the Submit button on the top right corner, to submit the PSUR:

Click Complete action in the pop-up window to finish the process:
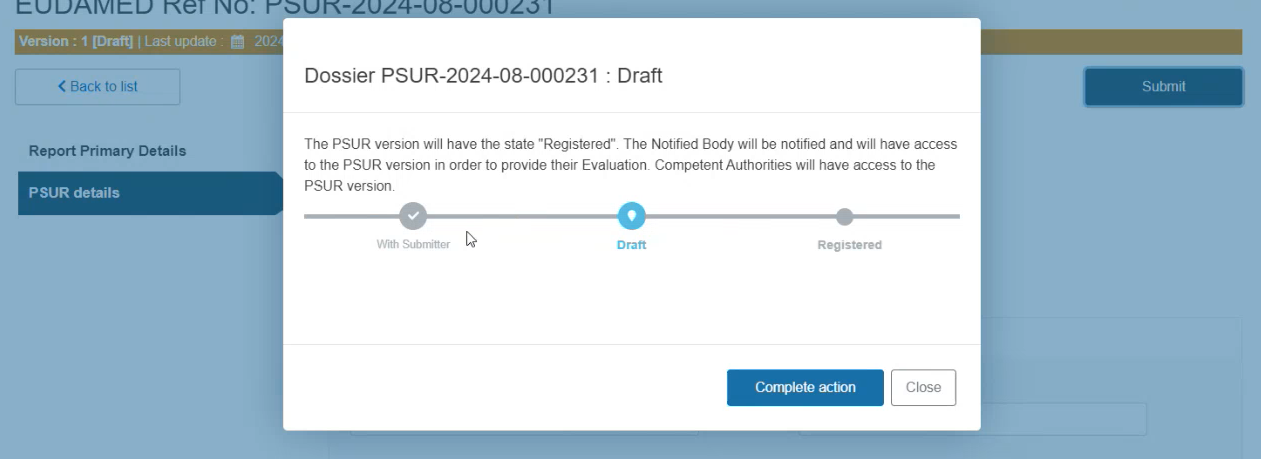
The system will redirect you to the Report Primary Details screen of the submitted PSUR in preview mode.
Note
After submitting a PSUR or creating a new version, for users with LAA profile only, CAs and NBs referenced in the report will receive a notification in their Notifications inbox (Information tab):