View details of a registered UDI-DI/EUDAMED ID
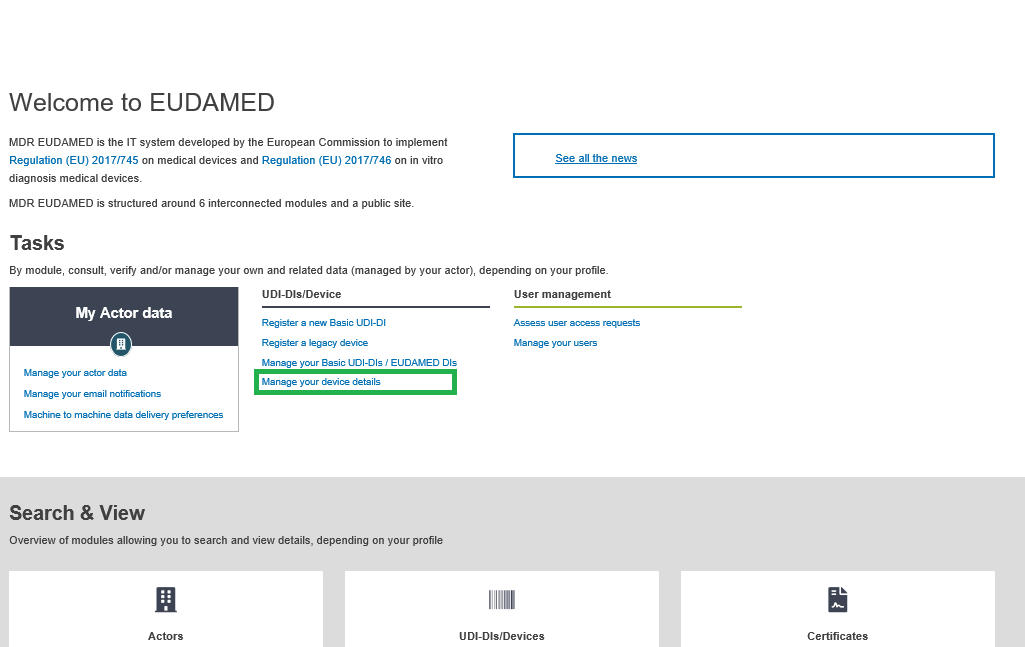
On the dashboard, click on Manage your device details:
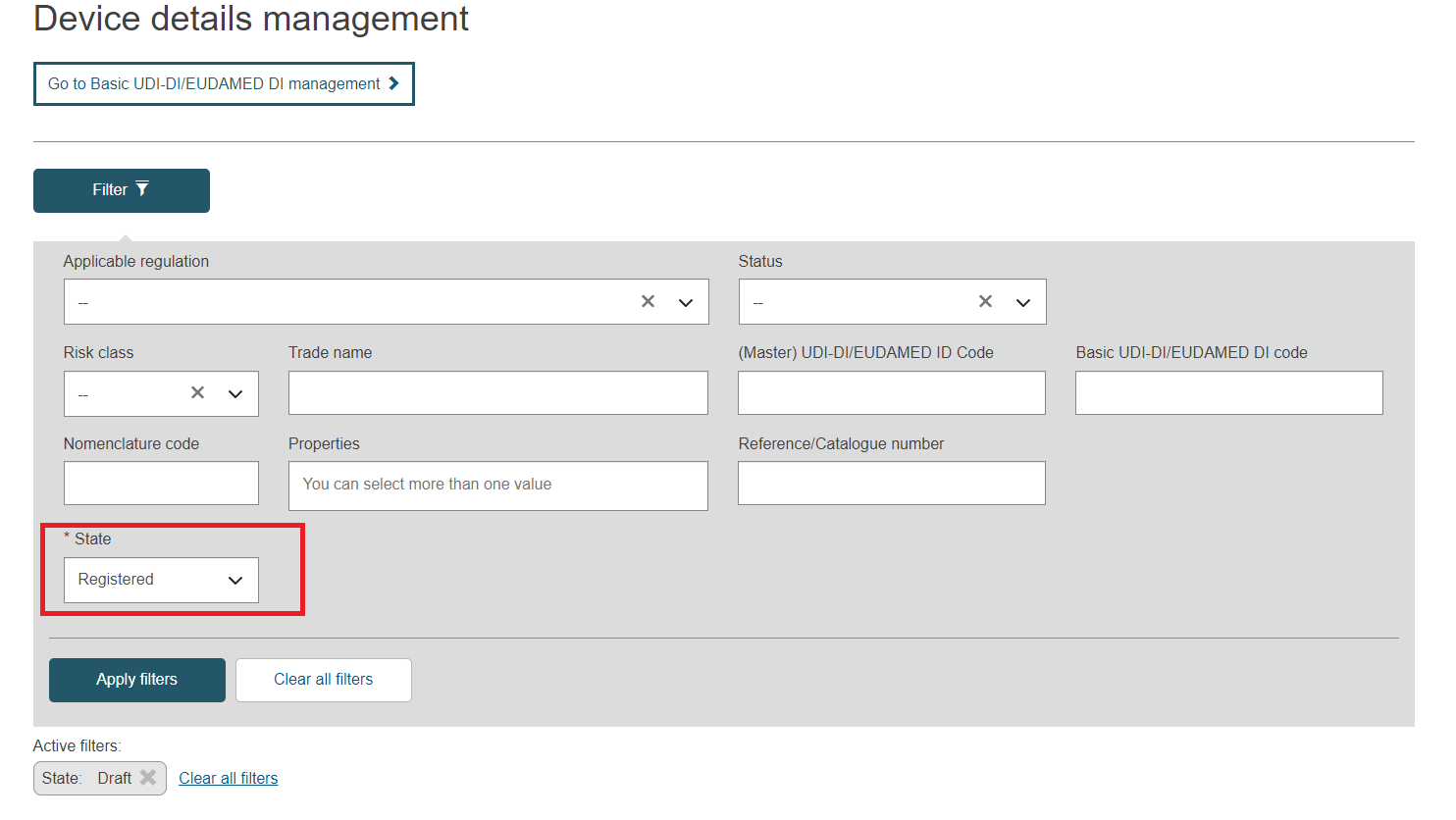
Select the option Registered in the State field and click on Apply filters:
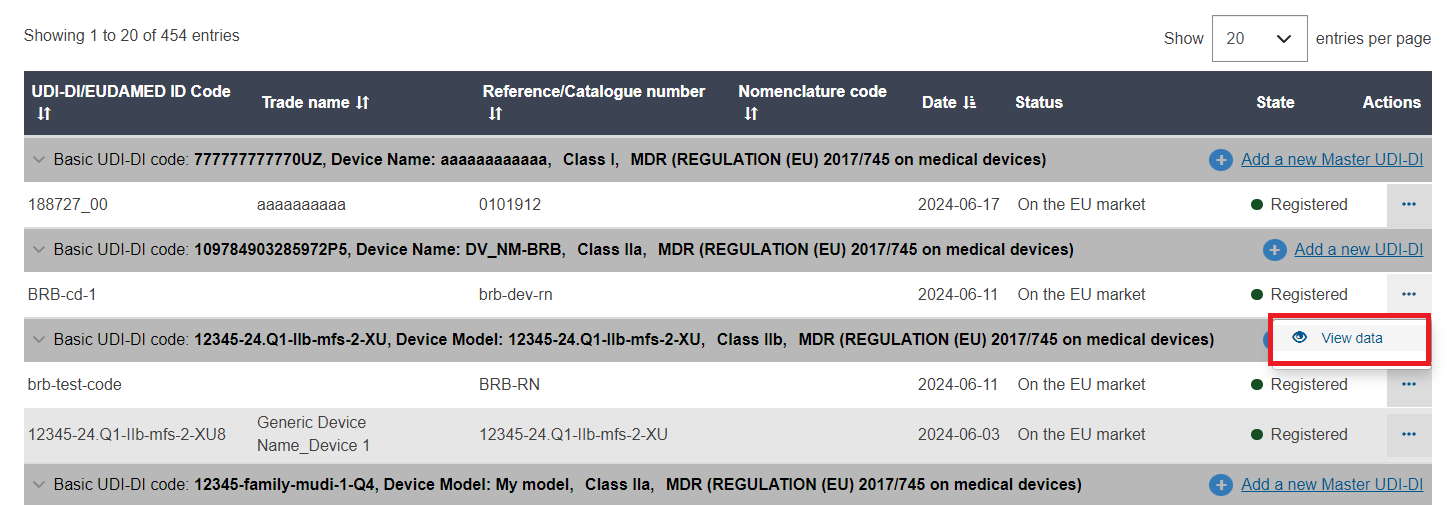
A list of devices will be displayed. Click on View data under the three dots of the desired entry:
You will see a summary of your device details, divided into the following subsections:
UDI-DI details:
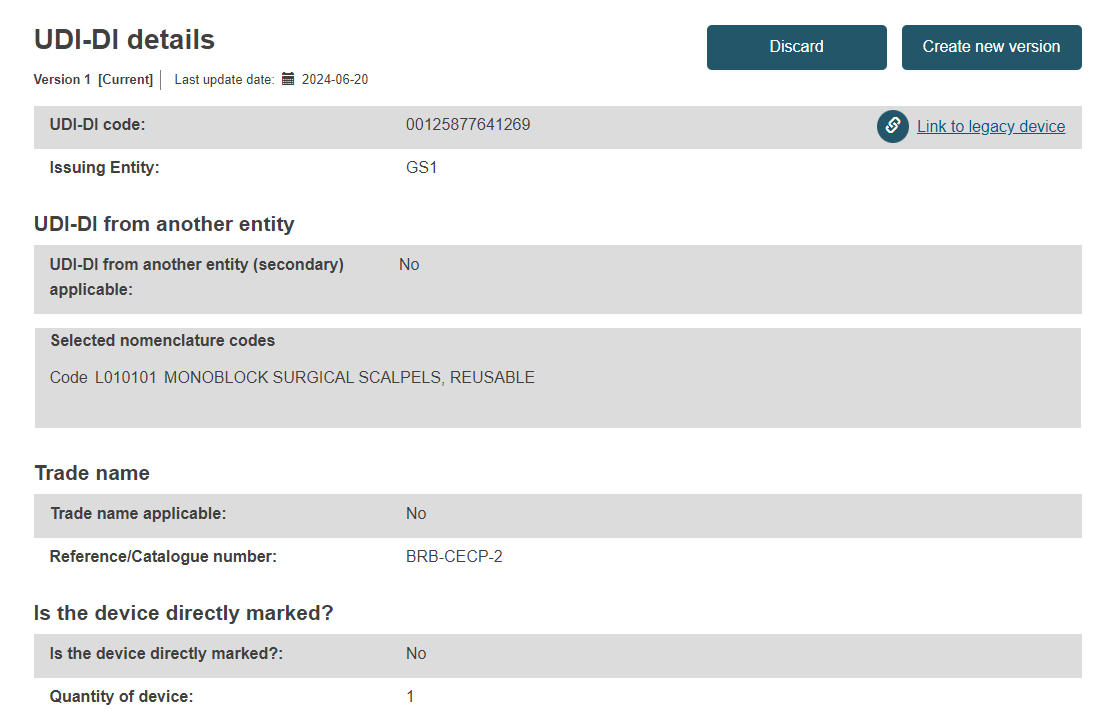
Note
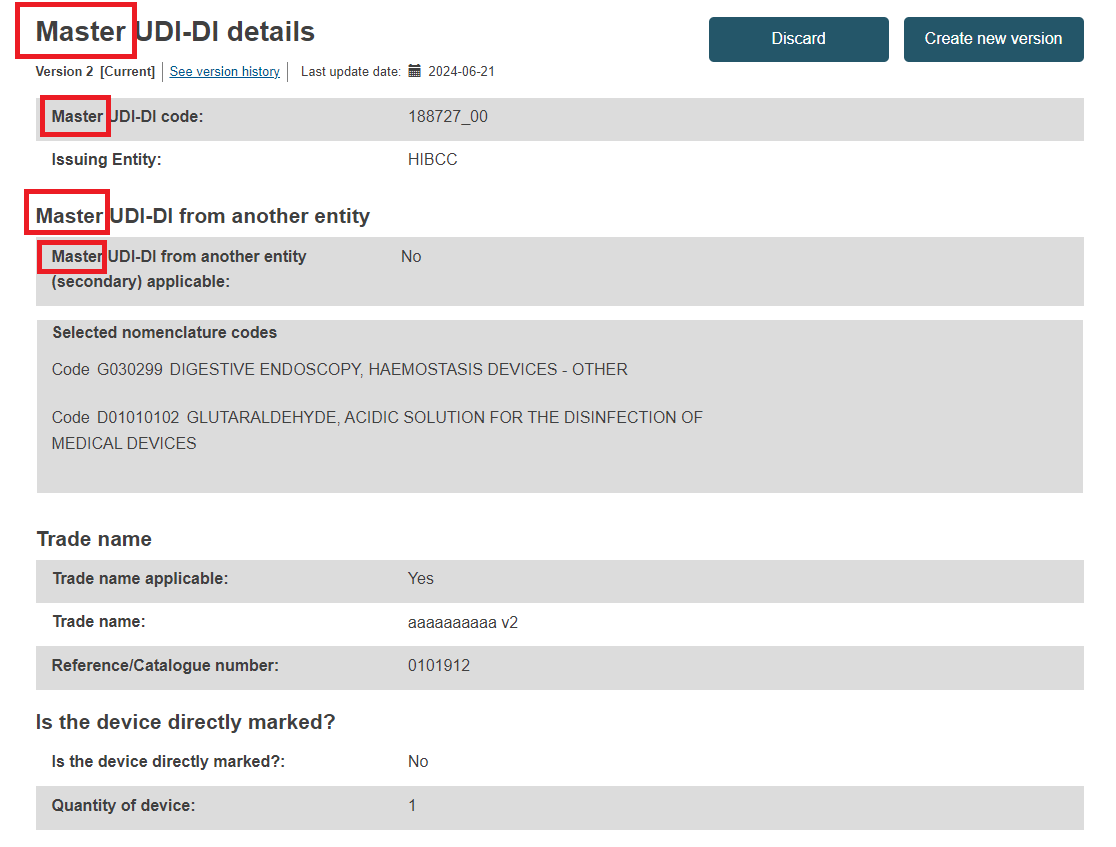
Master UDI-DI Details section for a registered Master UDI-DI:
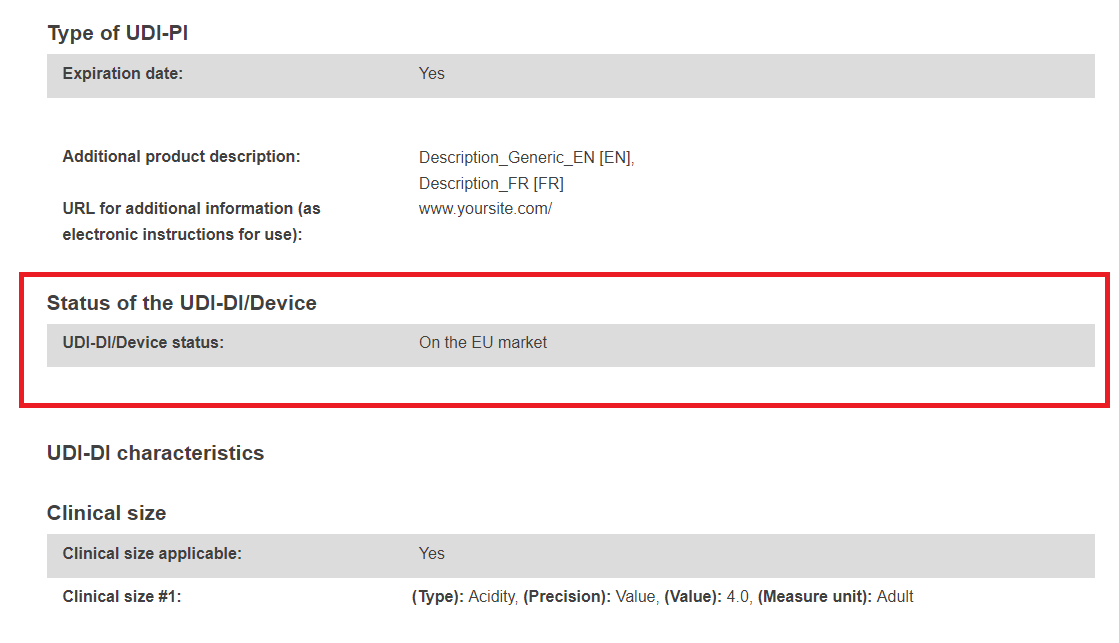
The UDI-DI/Device sub-status subsection will become visible under the UDI-DI/Device status subsection once a final Field Safety Notice (FSN – Vigilance module) has been registered for the selected UDI-DI/EUDAMED ID referenced in the corresponding Field Safety Corrective Action (FSCA – Vigilance module).
Note
The sub-status of the device will be set to Field safety corrective action initiated if any of the following manufacturer actions are selected in the corresponding FSCA:
IFU or labeling change
Software Upgrade
On-site modification/inspection by
Customer information only
Other
The sub-status of the device will be set to Recalled if any of the following manufacturer actions are selected in the corresponding FSCA:
Product Removal - Partial Recall (Lot/Batch/Model)
Product Removal - Full Recall

Note
When the FSCA status transitions to Action completed, the system will remove the corresponding device sub-status:
If the FSCA status referencing the device transitions to In progress, the sub-status will be displayed again.
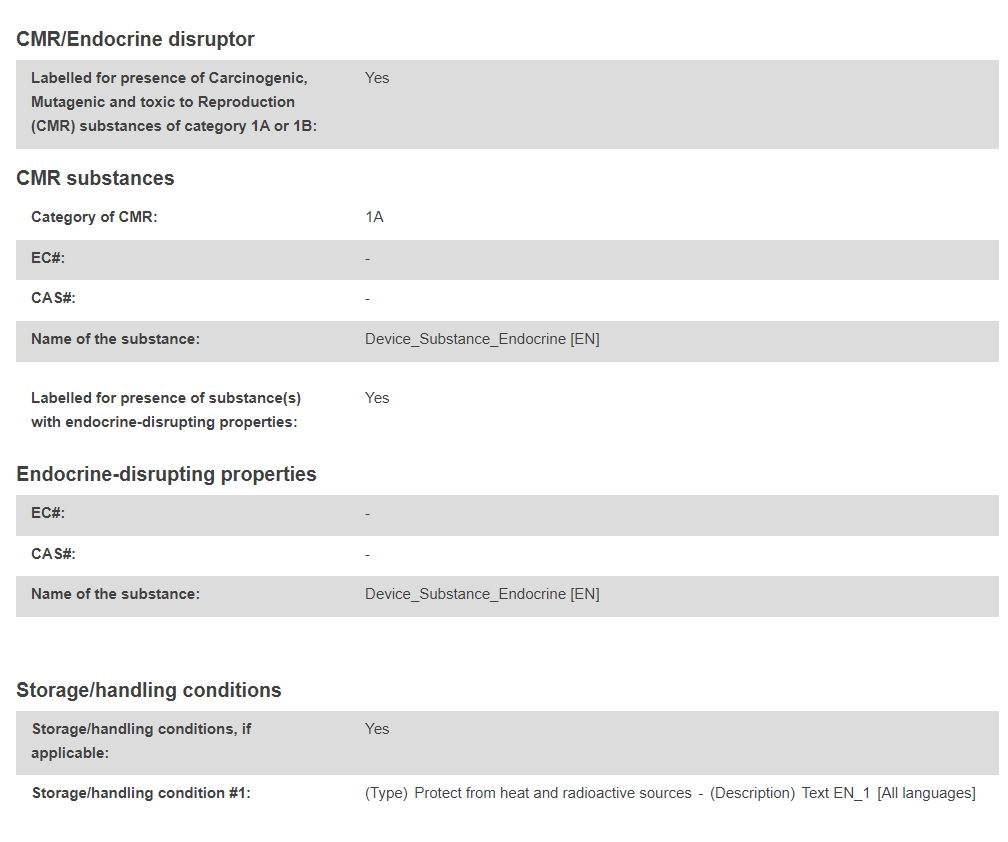
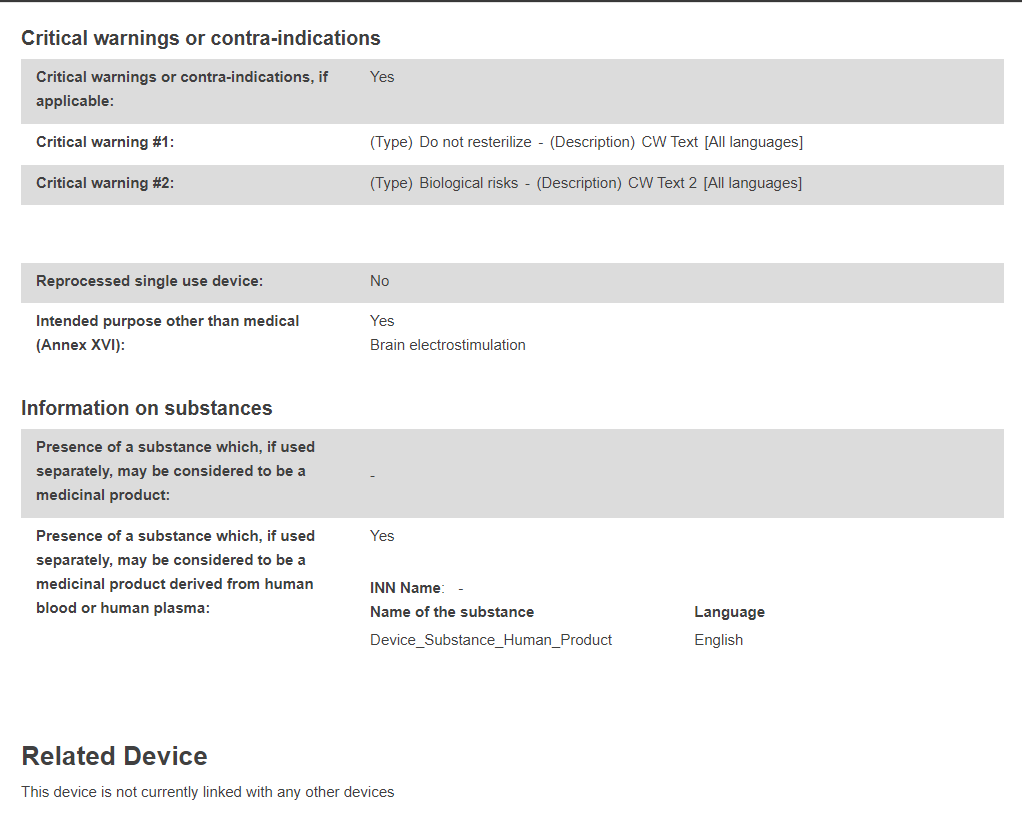
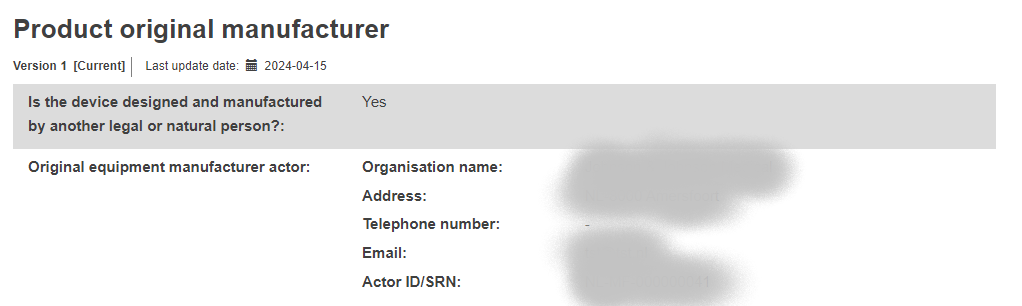
Product original manufacturer:
Market Information:

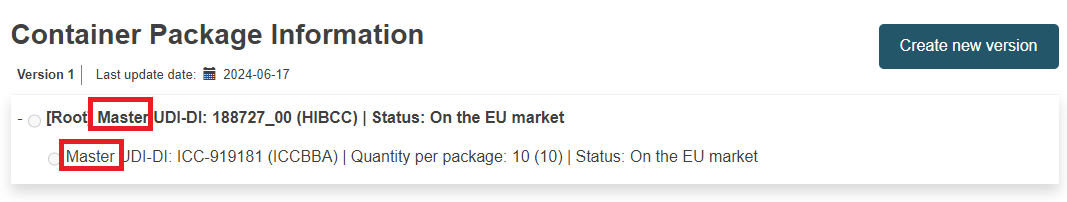
Container Package Information:
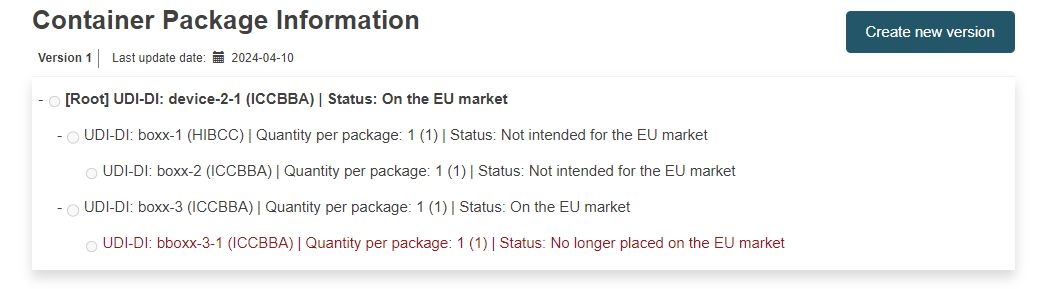
Note
Container Package Information section for a registered Master UDI-DI: