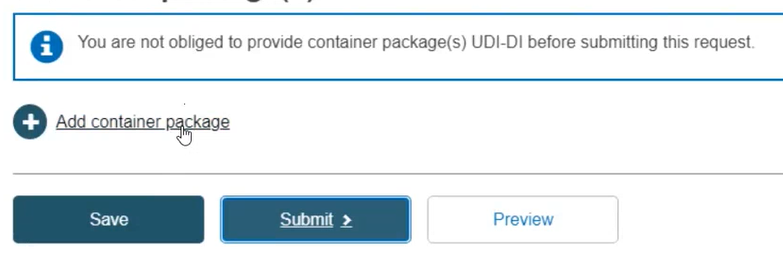
Container package details
 VIDEO: UDI carrier placing
VIDEO: UDI carrier placing
Click on Add container package when there is a higher packaging level for the root UDI-DI:
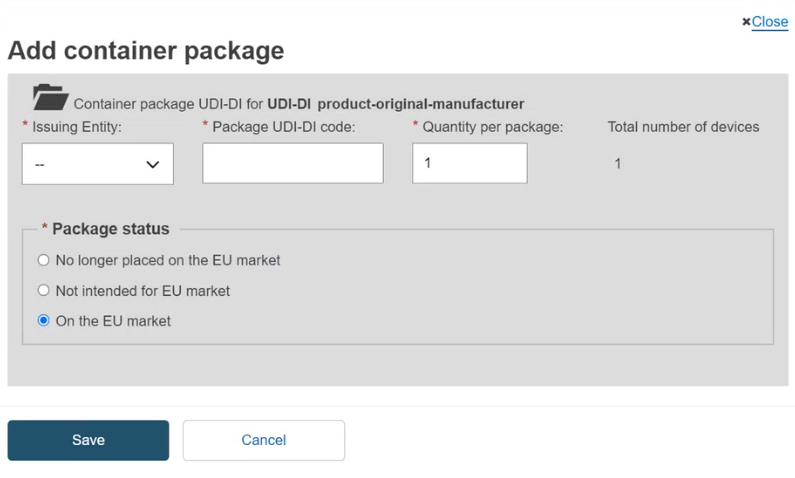
Each package level requires a unique UDI-DI assignment.
Start by registering the container package associated with the root UDI-DI (also known as the primary UDI-DI). You may add multiple levels and container packages. Input the Issuing Entity, UDI-DI code for the package, Quantity per package, select the Package status and then click Save:
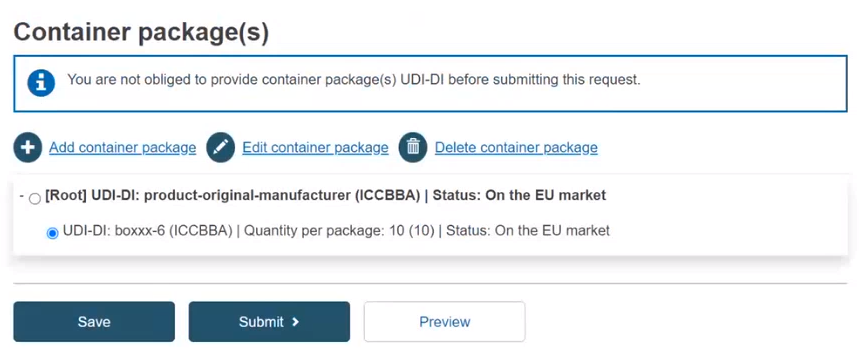
Note
If the UDI-DI already exists in EUDAMED, the system will prevent you from saving.
Note
If the status of the device for this container package is either No longer placed on the EU Market or Not intended for the EU Market, the Package status options are greyed out and any container package added to this device will automatically have the same Package status as the device.
Note
When registering a Master UDI-DI code, a specific format validation algorithm is applied when the issuing entity is GS1:
For Standard soft contact lenses and Standard Rigid Gas Permeable (RGP) contact lenses the system applies the GMN format validation algorithm.
For Made-to-order soft contact lenses and Made-to-order Rigid Gas Permeable (RGP) contact lenses the system applies the GTIN format validation algorithm.
Select the generated information and click on Submit:
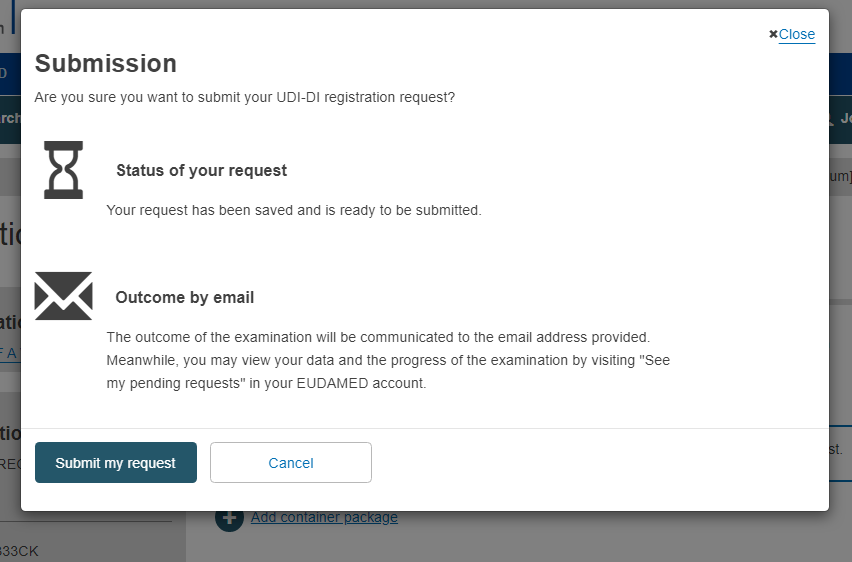
Confirm your submission in the pop-up window:
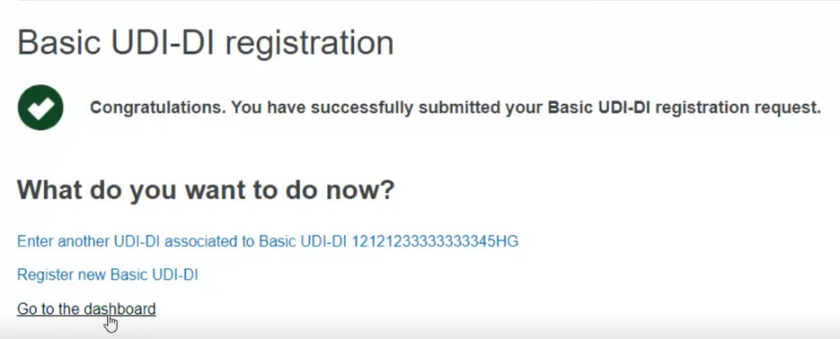
The screen will display a success message:
Important
After submitting the Device, the state of the Device (Basic UDI-DI and UDI-DI) will be:
Registered, if the Basic UDI-DI data does not require confirmation from the Notified Body (Basic UDI-DI and UDI-DI is publicly available in the EUDAMED public website);
Submitted, if the Basic UDI-DI data requires confirmation from the Notified Body (Basic UDI-DI and UDI-DI is not publicly available and will only get the Registered state and become publicly available after Notified Body confirmation).