Device identification information
EUDAMED will display the identifier of the Device (the previously provided UDI-DI or the EUDAMED ID generated based on the provided/generated EUDAMED DI. EUDAMED ID has the same code as the EUDAMED DI, except that it is with a “D-“ prefix instead of the “B-“ prefix):
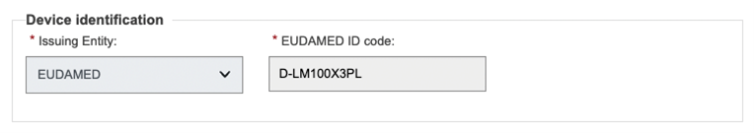 |
Enter the EMDN code. Click on Find and select the correct one:

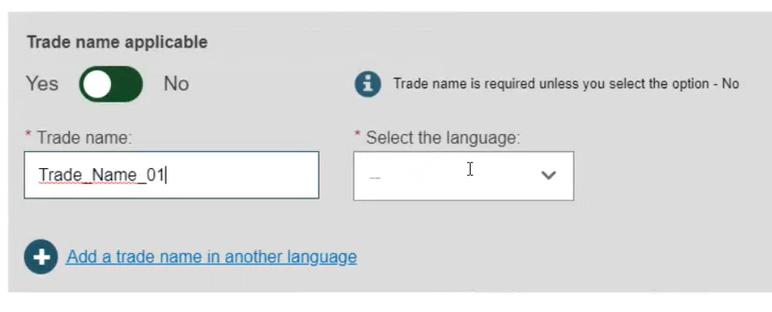
If applicable, enter the trade name and select the language, otherwise select No:
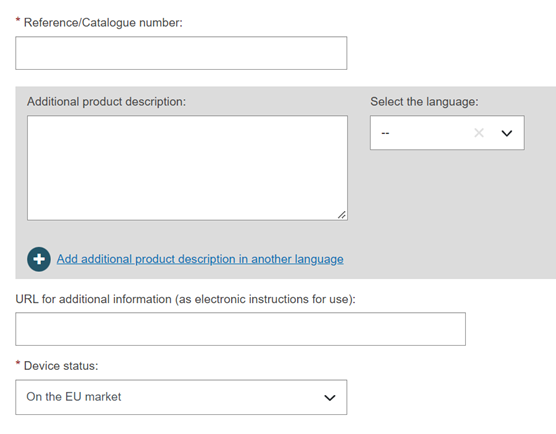
Enter a reference/catalogue number and any additional information you might have:
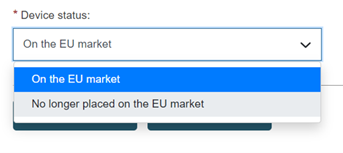
Choose the market status of the Device: