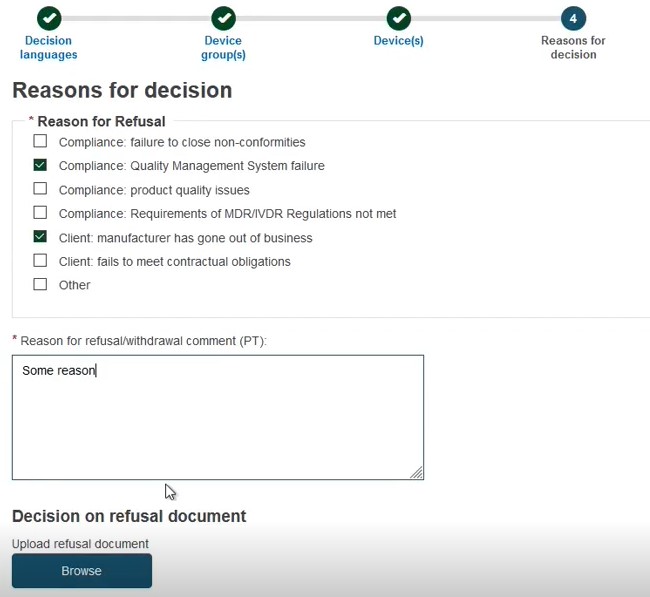
Reasons for the decision
Select one, or multiple, reason(s) for the refusal/withdrawal decision and provide a comment. The Decision on refusal document is optional:
 |
You may preview your choice, or click Submit:
 |
A warning message displays.
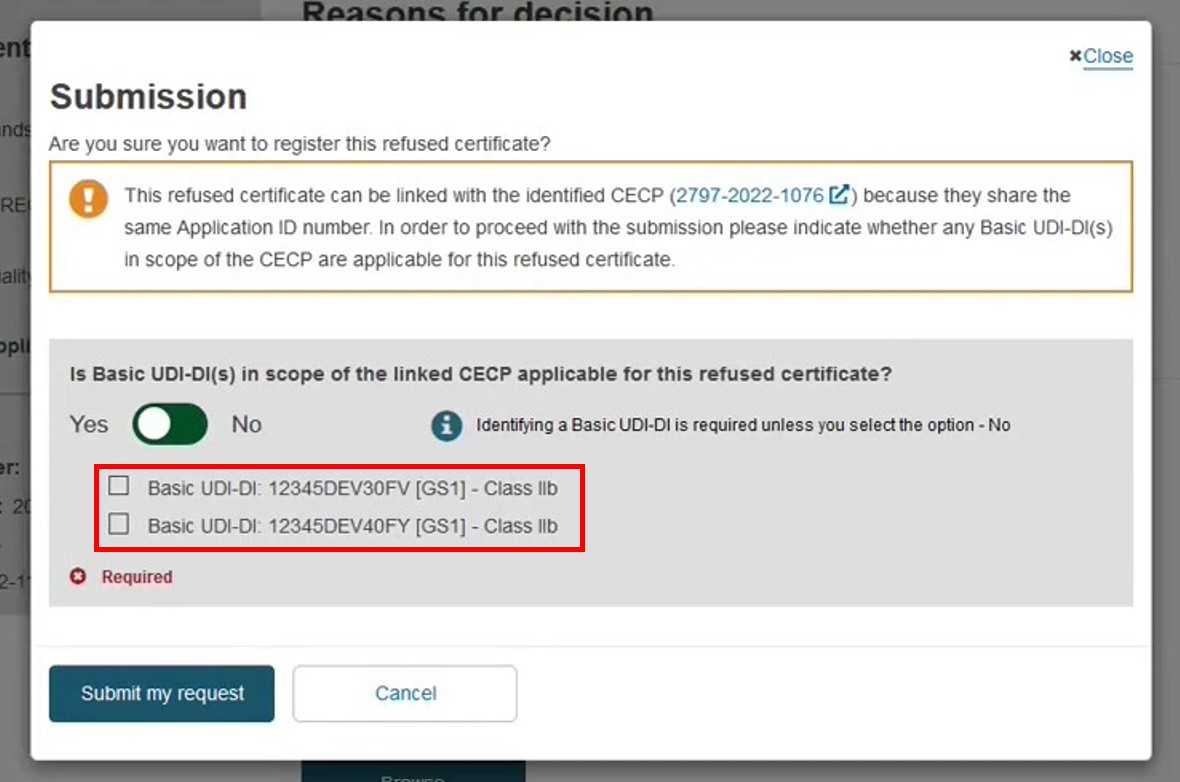
In case a CECP has been registered for the same conformity assessment application then the system will display an information pop-up with the identified CECP. The CECP record can be viewed by opening the provided link  . For Quality-type certificates, the NB had the option not to provide the Basic UDI-DI, but here they can indicate to which device(s) the refused certificate refers.
. For Quality-type certificates, the NB had the option not to provide the Basic UDI-DI, but here they can indicate to which device(s) the refused certificate refers.
In this case, if you select both Basic UDI-DIs, the final CECP status will become Refused certificate. If you select one, the status will become Refused certificate partially, until the remaining device(s) is registered to an issued certificate. Then the status will become Issued refused certificate. See the CECP status here:
 |
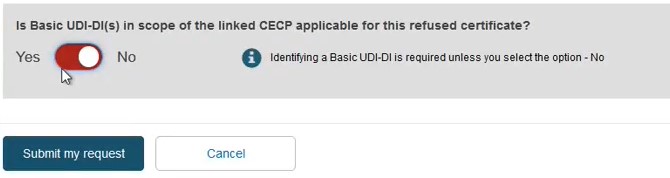
Alternatively, toggle to No if you choose not to link the CECP to the refused certificate:
 |
Click Submit my request. A confirmation message displays:
 |