Provision of device data
If the certificate also includes devices, indicate whether the certificate is for a Custom made class III implantable, if yes provide a description, if no provide:
The name and the risk class, the reference/catalogue number and the risk class or the Basic UDI-DI (Basic UDI-DIs must be already submitted by the manufacturer in EUDAMED).
In this step you may choose to provide a custom-made device by selecting Yes within Custom made class III implantable box:
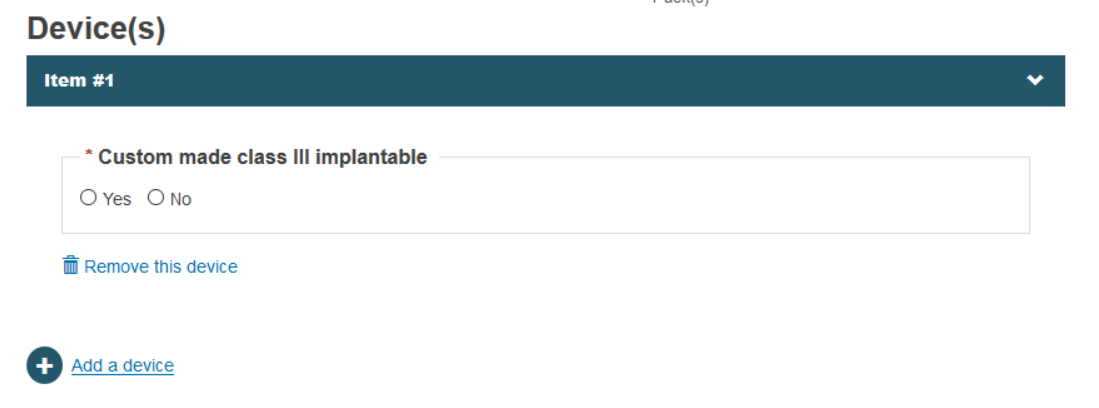
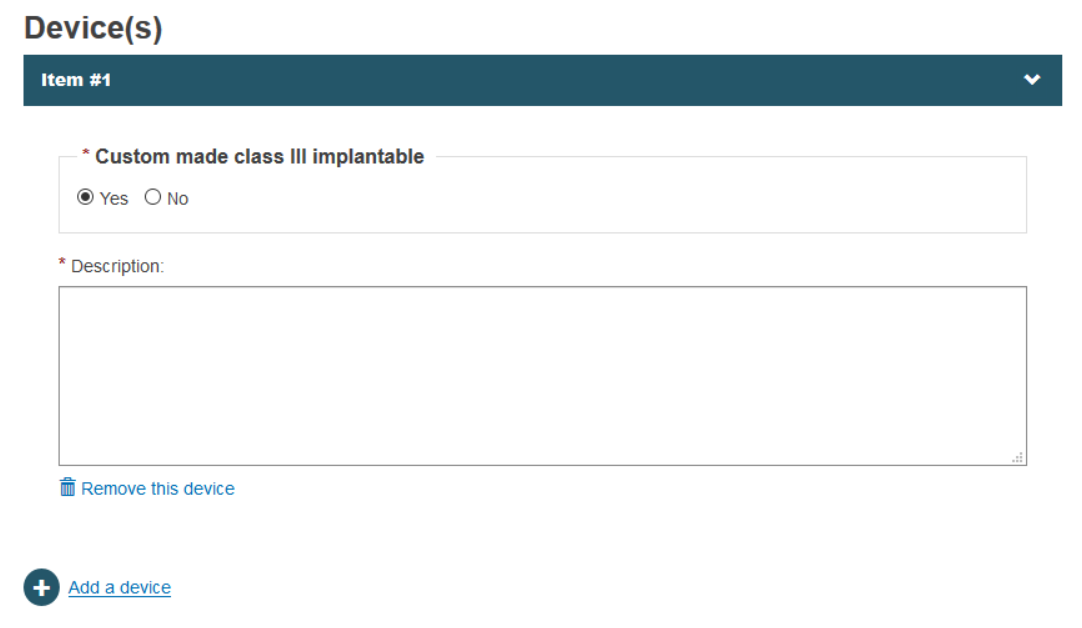
By doing so, EUDAMED will allow you to provide a description for the custom-made device:
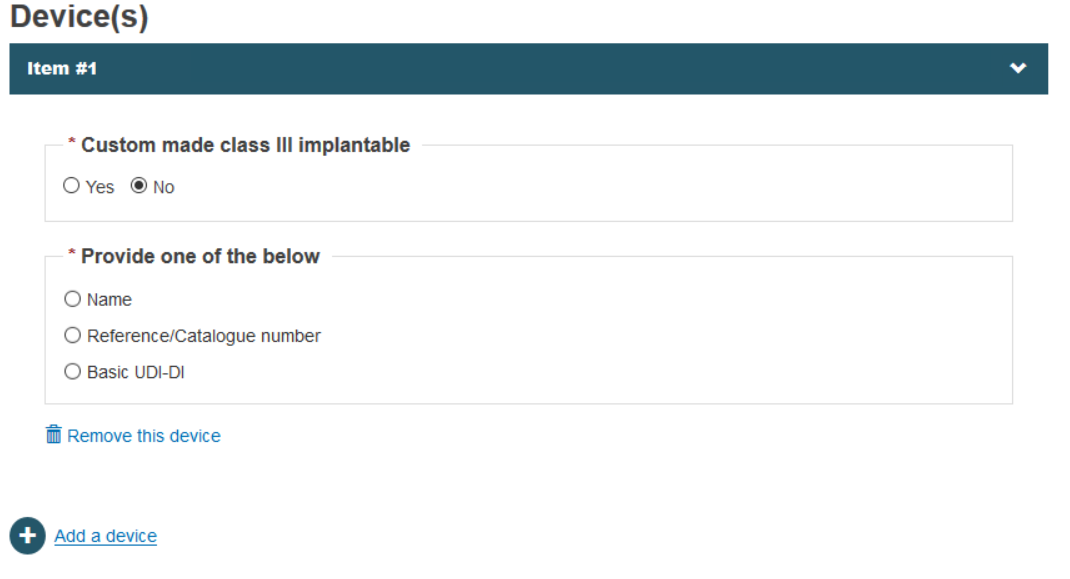
When you select No, then the system will provide a dialog to select Name or Reference/Catalogue number options in order to register a device by its name, its reference/catalogue number or its basic UDI-DI:
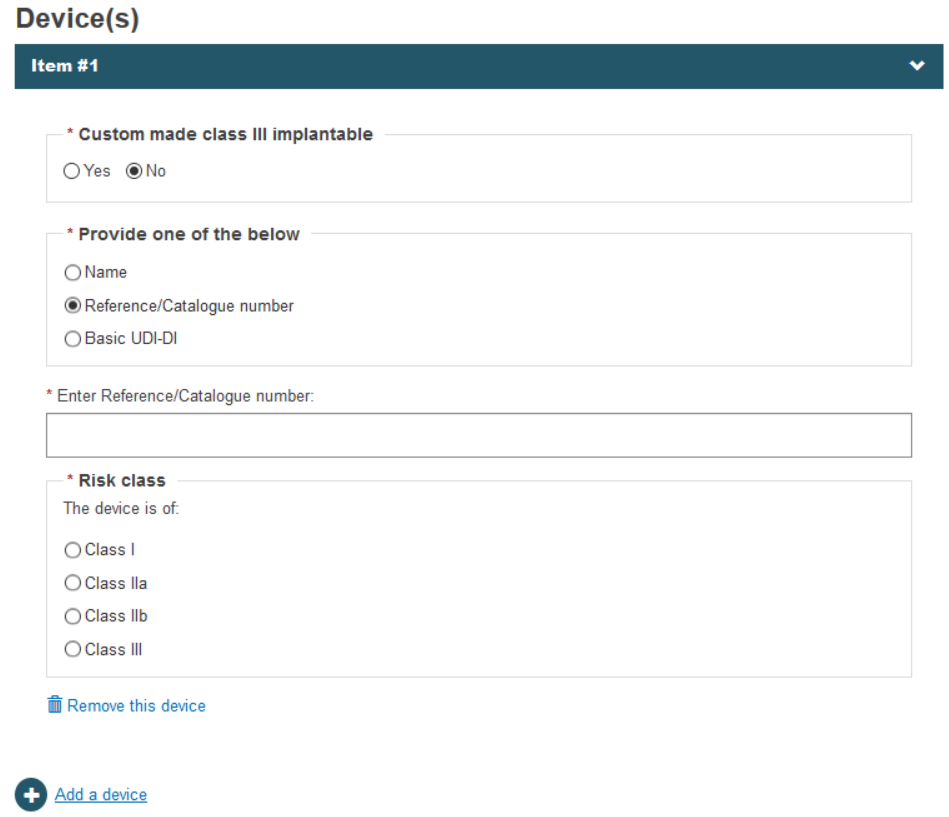
When either Name or Reference/catalogue number is selected, you must provide the risk class of the device:
Click Save to save your draft or Save & Next to continue to the next step.