Search and View CECP
You have the possibility to view all Clinical Evaluation Consultation Procedure (CECP) records.
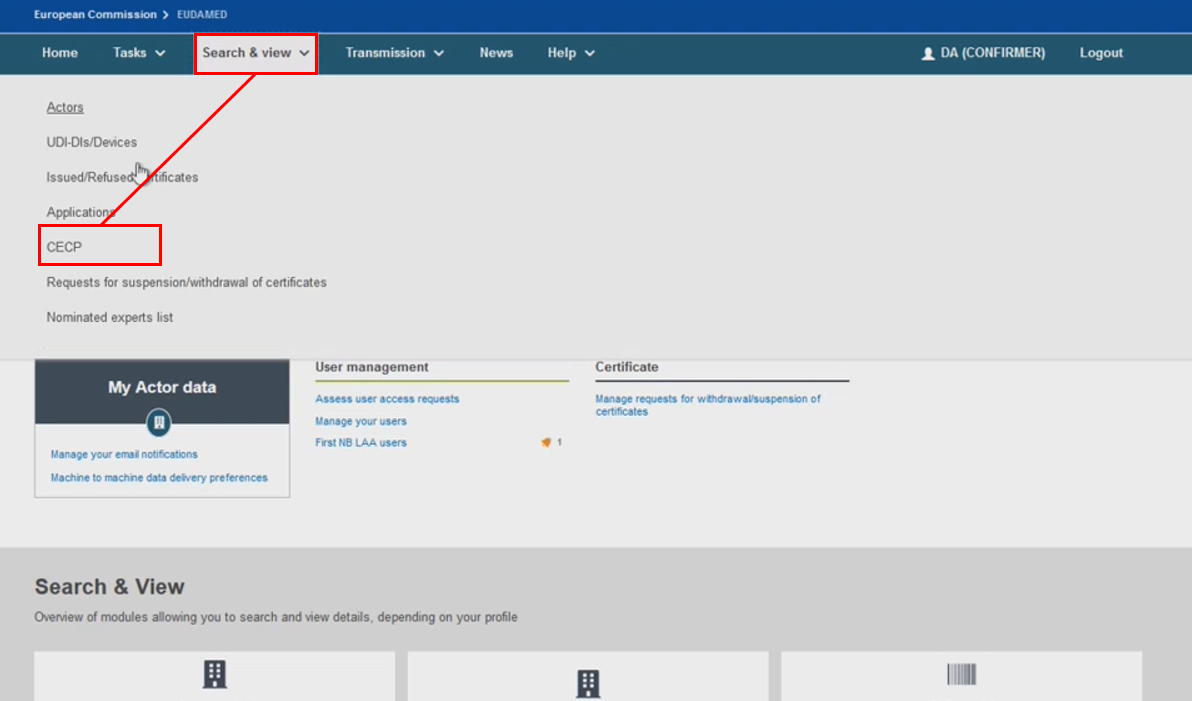
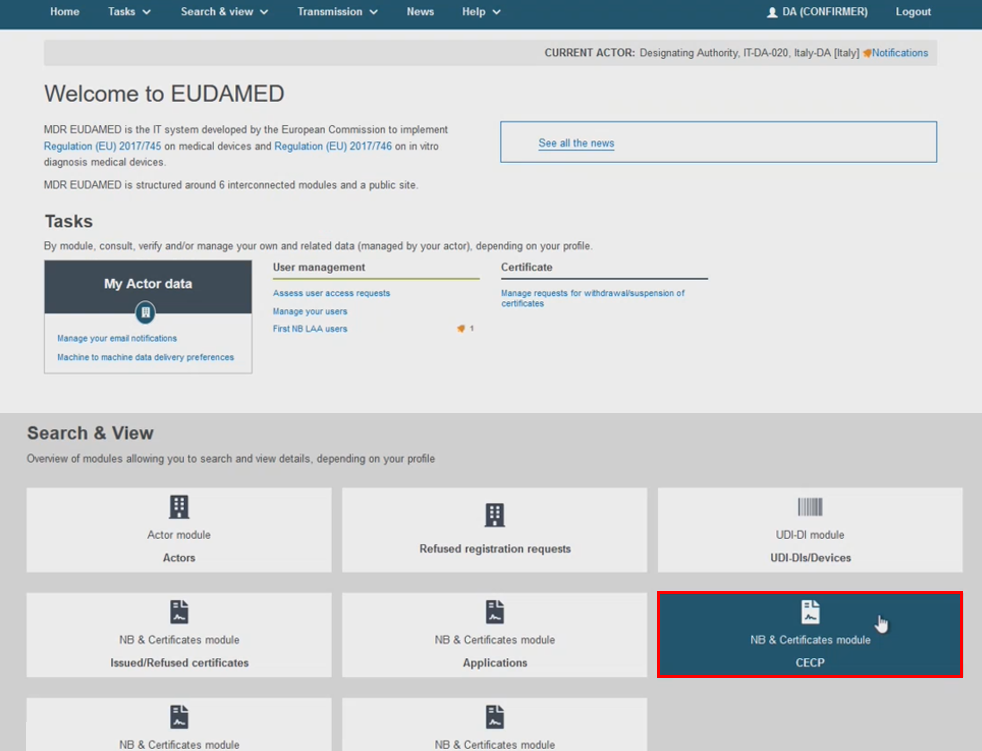
Access the CECP search filters from the homepage on the header menu by clicking Search & View, then click CECP. Alternatively, use the CECP button available in the Search & View dashboard:
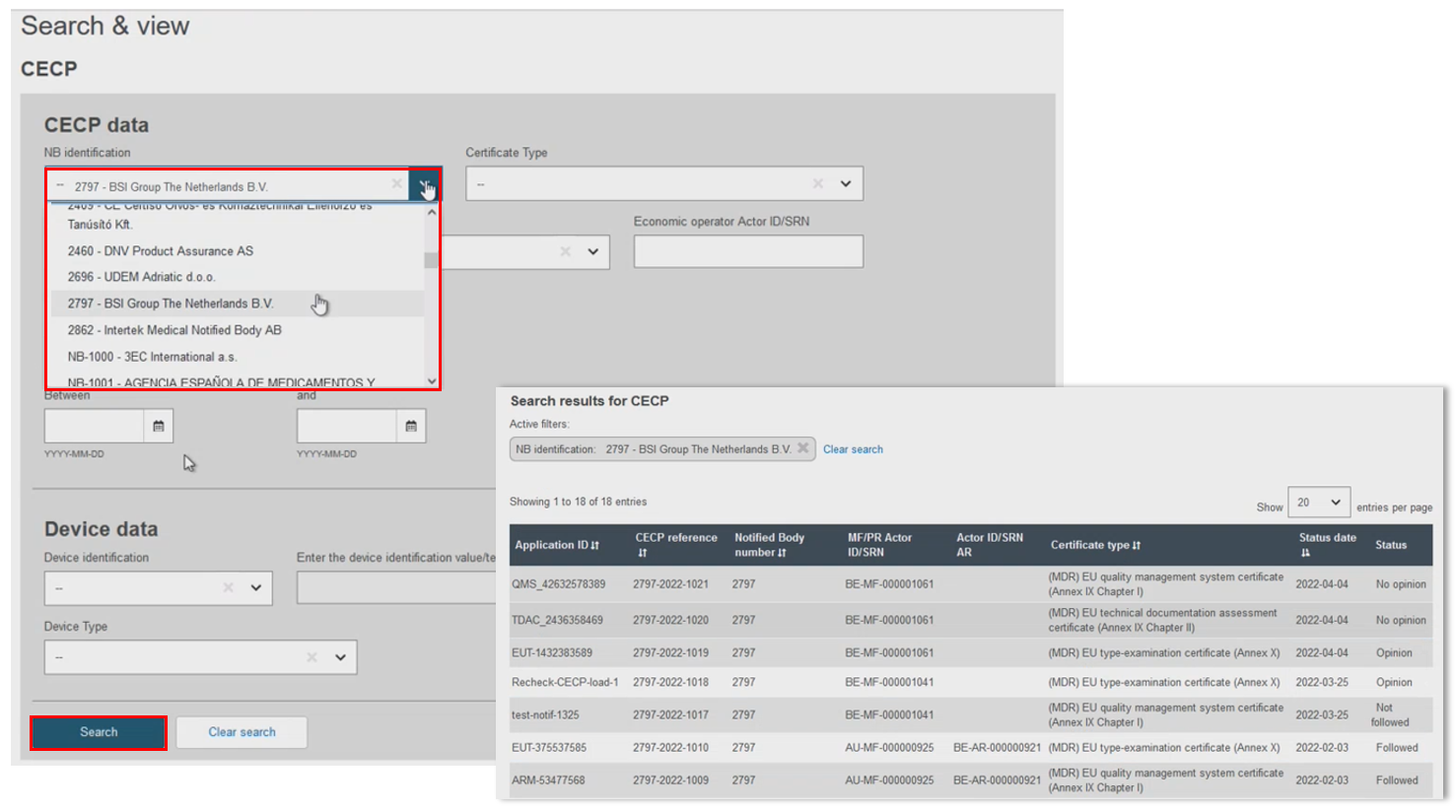
Complete the search filters to find the intended CECP record, then click Search:
The Notified Body (NB) filter pulls all CECP records registered by that NB):
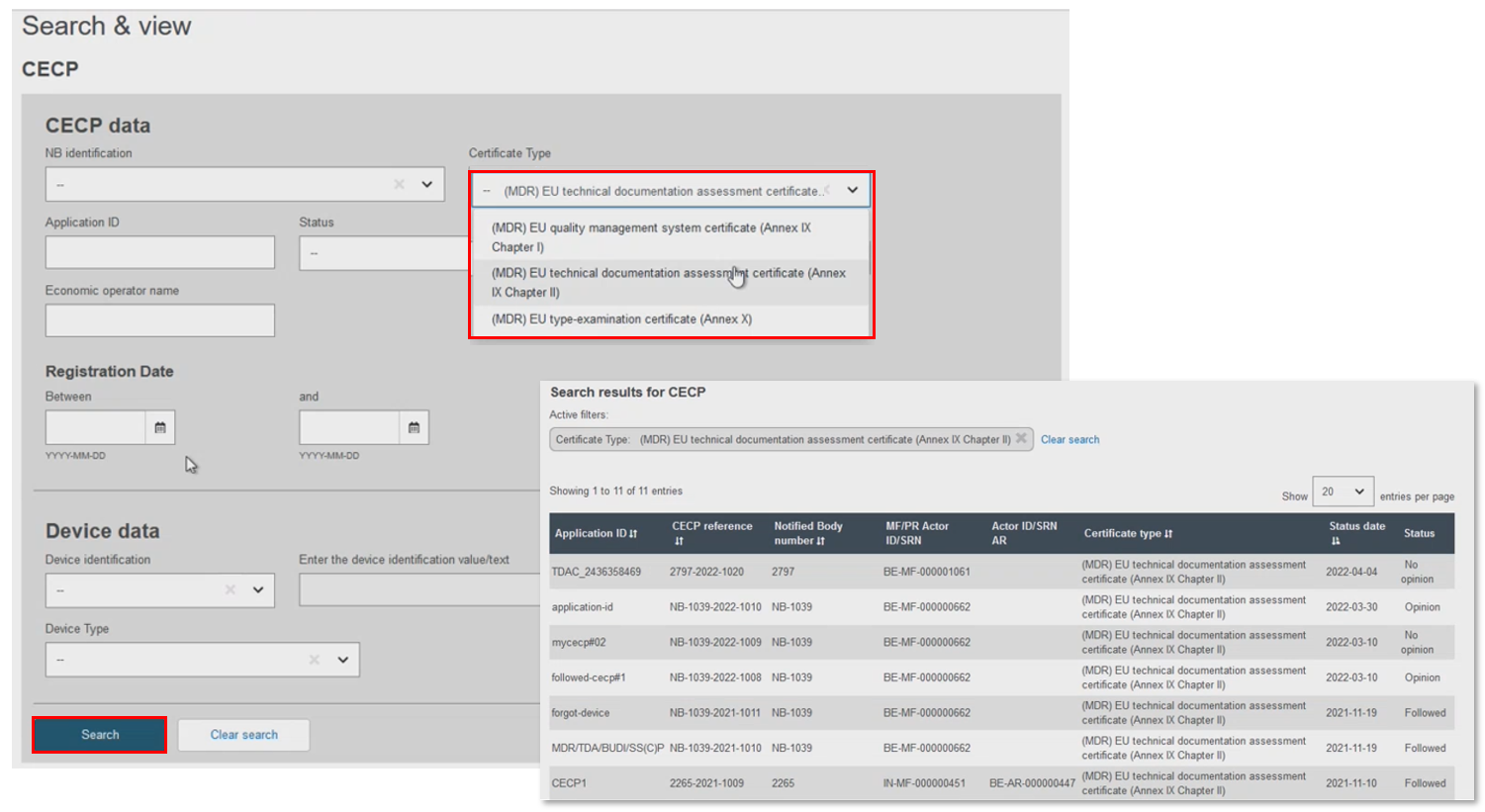
Filter results by Certificate Type (only three apply for devices requiring CECP):
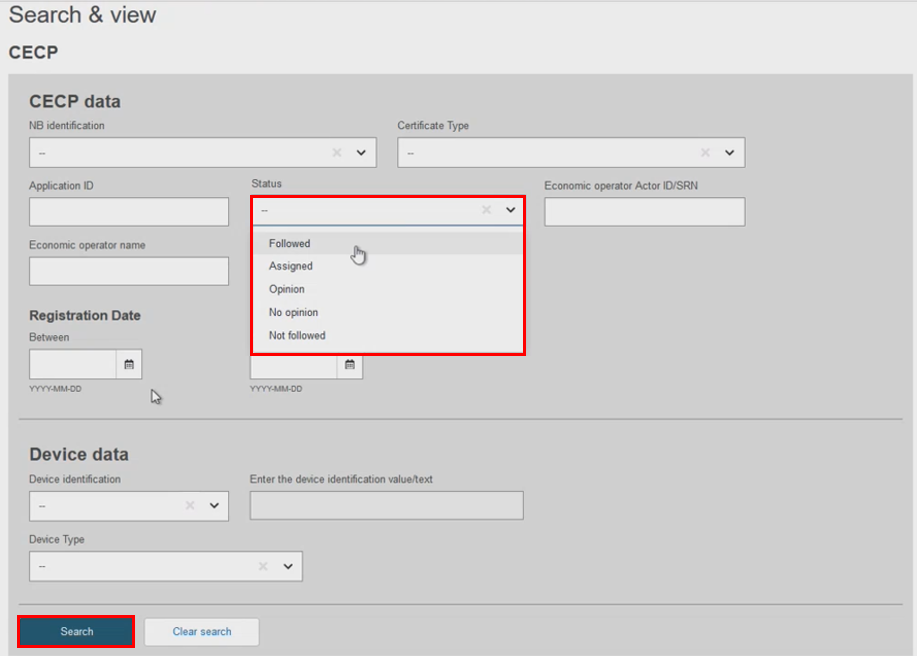
Search using the CECP Application ID or the Status filters. Note that the Status filter contains the following values:
Followed – Notified Body follows the CECP.
Assigned – EC user assigned a Rapporteur and Co-rapporteur for this CECP case.
Opinion – The screening panel decided to provide their opinion on this CECP case. The status is changed when the screening panel provides their decision.
No opinion – The screening panel decided not to provide any opinion on this CECP case. The status is changed when the screening panel provides their decision.
Issued-certificate-partially – This status occur when a CECP references at least two (2) Basic UDI-DIs and there is at least one Basic UDI-DI that is not referenced in the issued certificate.
Refused-certificate-partially – This status occur when the CECP references at least two (2) Basic UDI-DI(s) and there is at least one Basic UDI-DI that is not referenced in the refused certificate.
Withdrawn-application – Final status. When the CECP is linked to a withdrawn application.
Refused-certificate – Final status. When all Basic UDI-DIs within the CECP are being referenced in a refused certificate.
Issued-certificate – Final status. When all Basic UDI-DIs within the CECP are being referenced in an issued certificate.
Issued-refused-certificate – Final status. When some Basic UDI-DIs within the CECP are being referenced in an issued certificate and other Basic UDI-DIs within the CECP are being referenced in a refused certificate.
Not-followed – Final status. Notified Body does not follow the CECP.
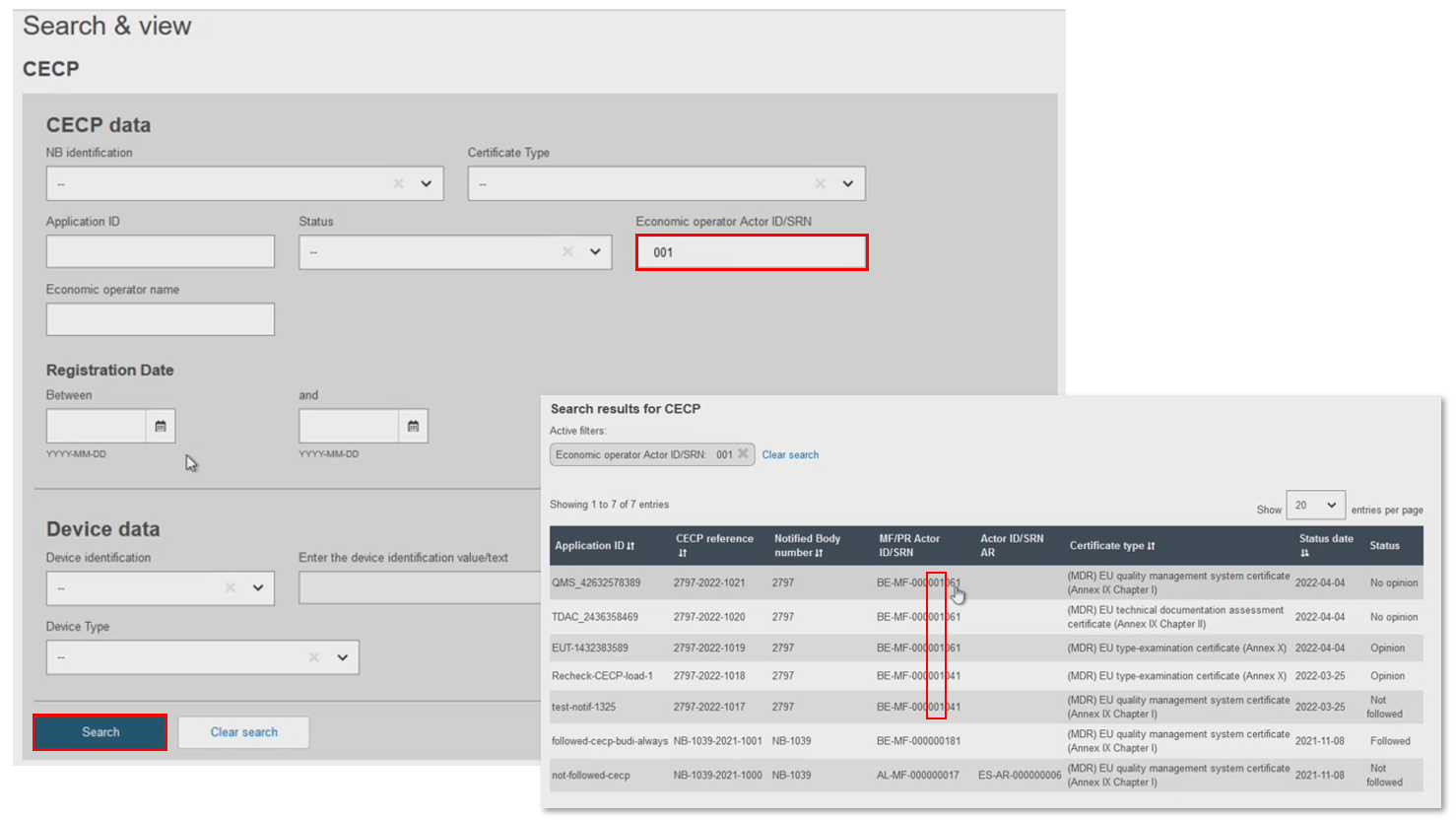
Filter by Economic operator Actor ID/SRN. The results show records with a matching Actor ID/SRN. Alternatively, just input the economic operator's Actor name, which again displays the matching Actor ID/SRN:
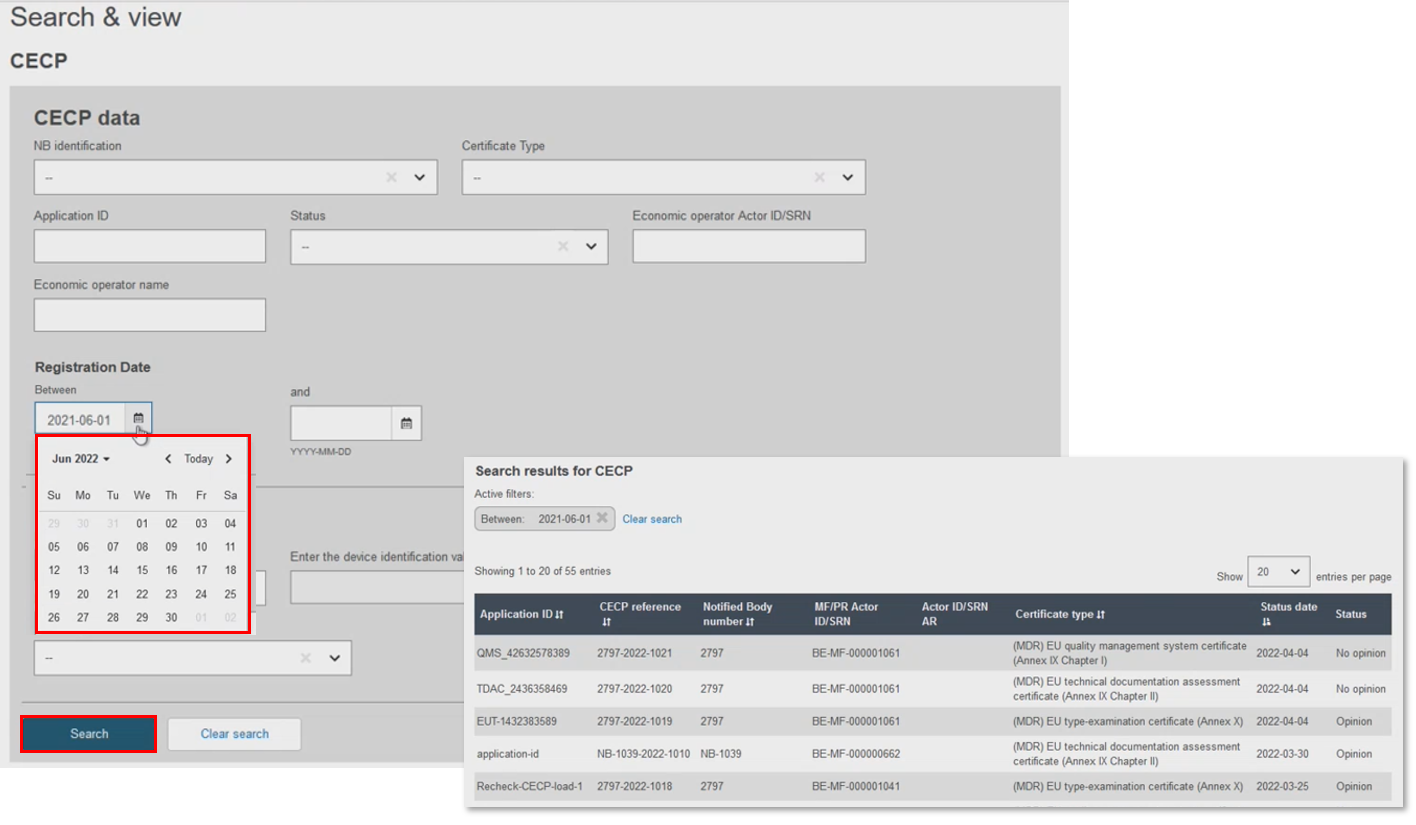
Filter by Date, using the registration date as a start date, or set a time period for the search. Use the calendar tool, or manually input the dates:
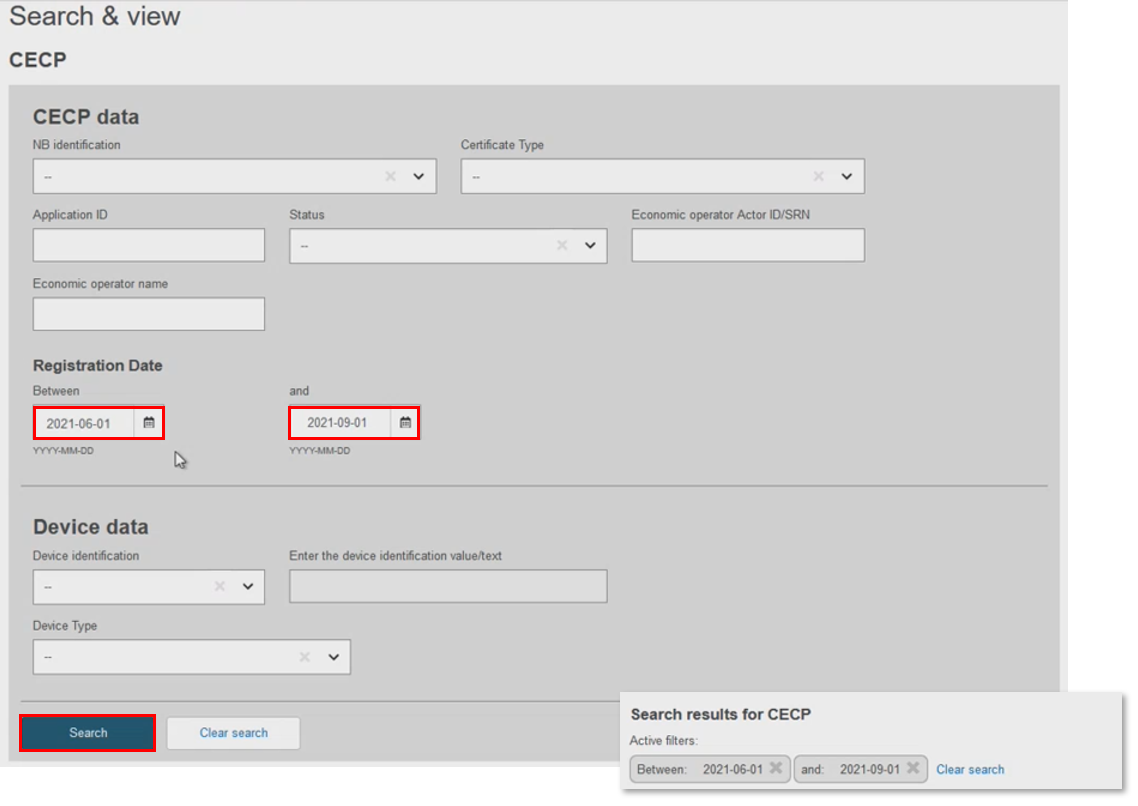
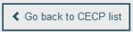
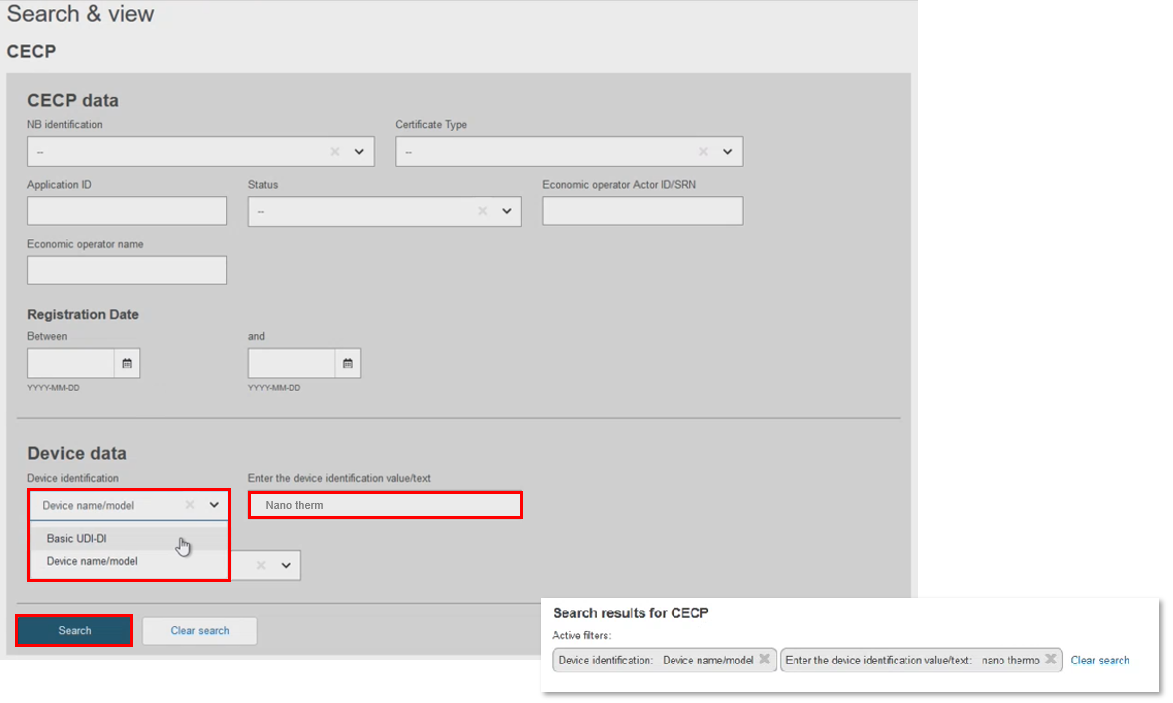
In Device Data, filter by Basic UDI-DI. There can only be one CECP record for each Basic UDI-DI, but there could be multiple Basic UDI-DIs in one CECP record. Select the record to display the CECP . Click
 to return to the search results:
to return to the search results:
Filter by Device name/model:
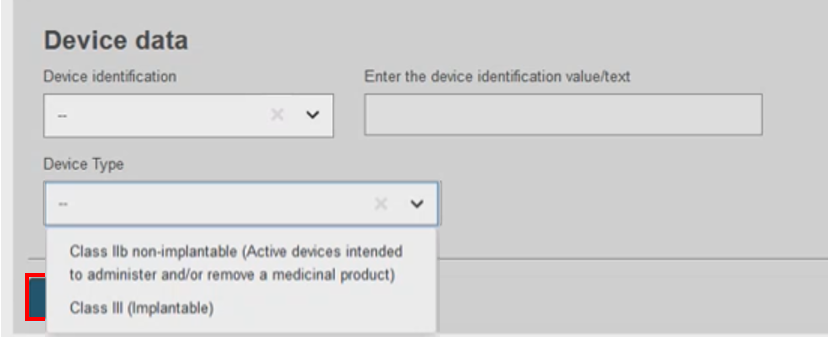
Filter by Device Type based on the device attributes:
The results dashboard displays a range of data identifying the device and CECP:
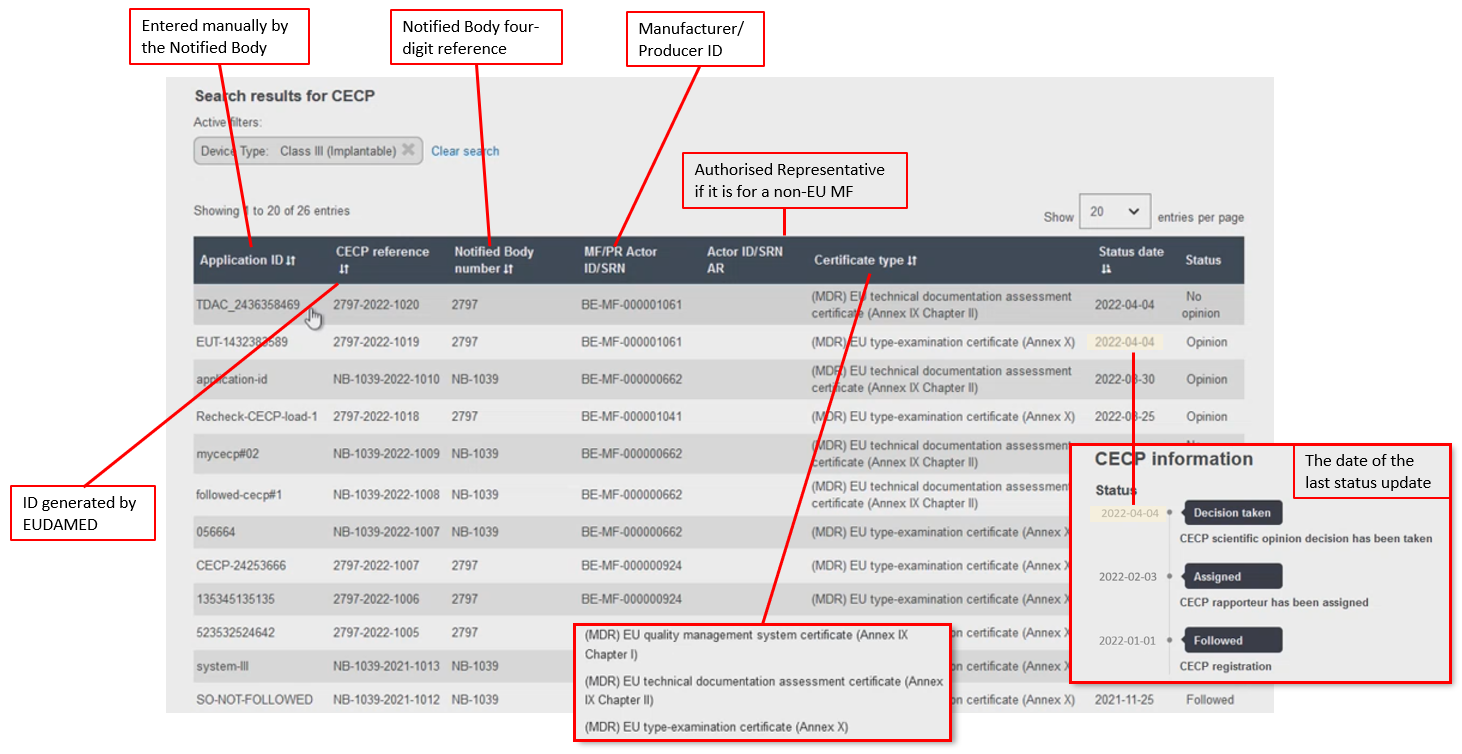
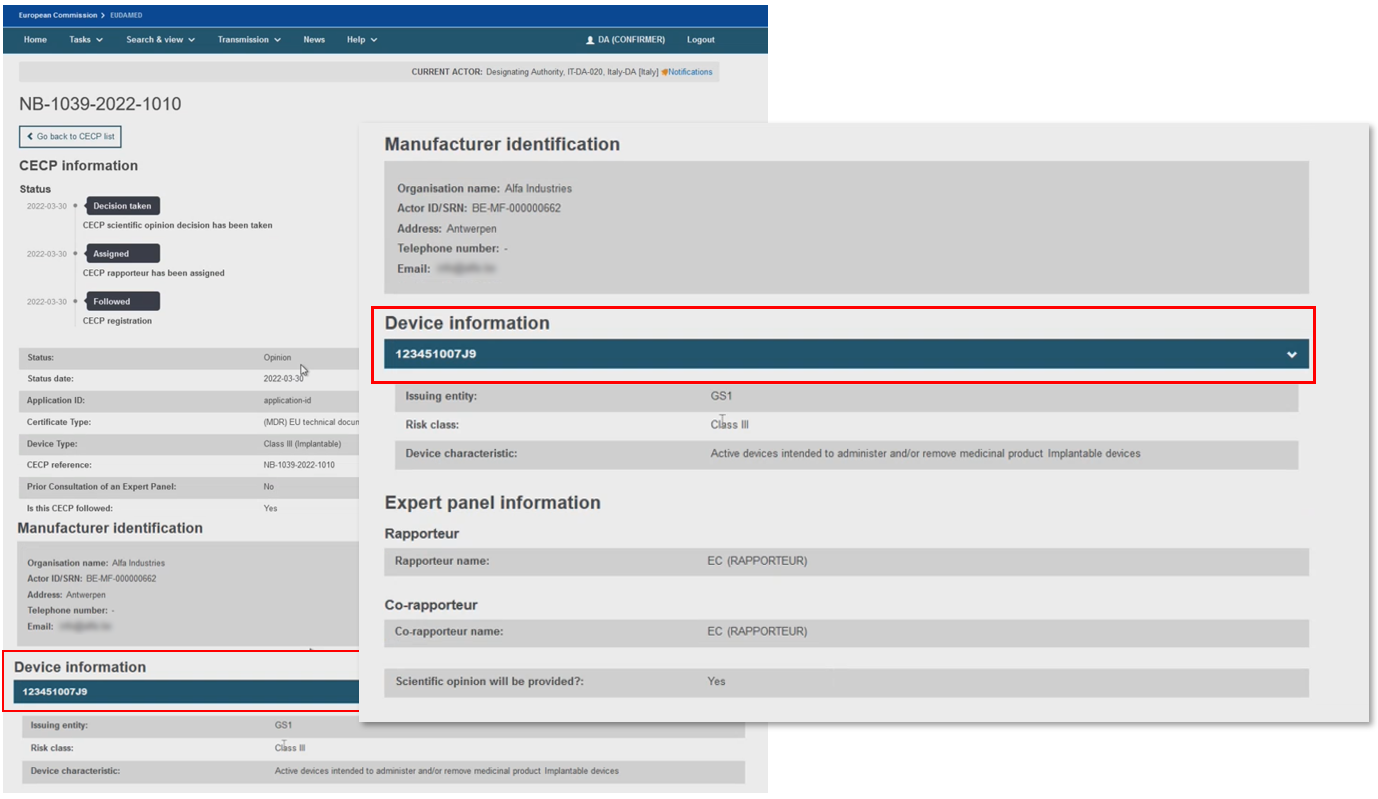
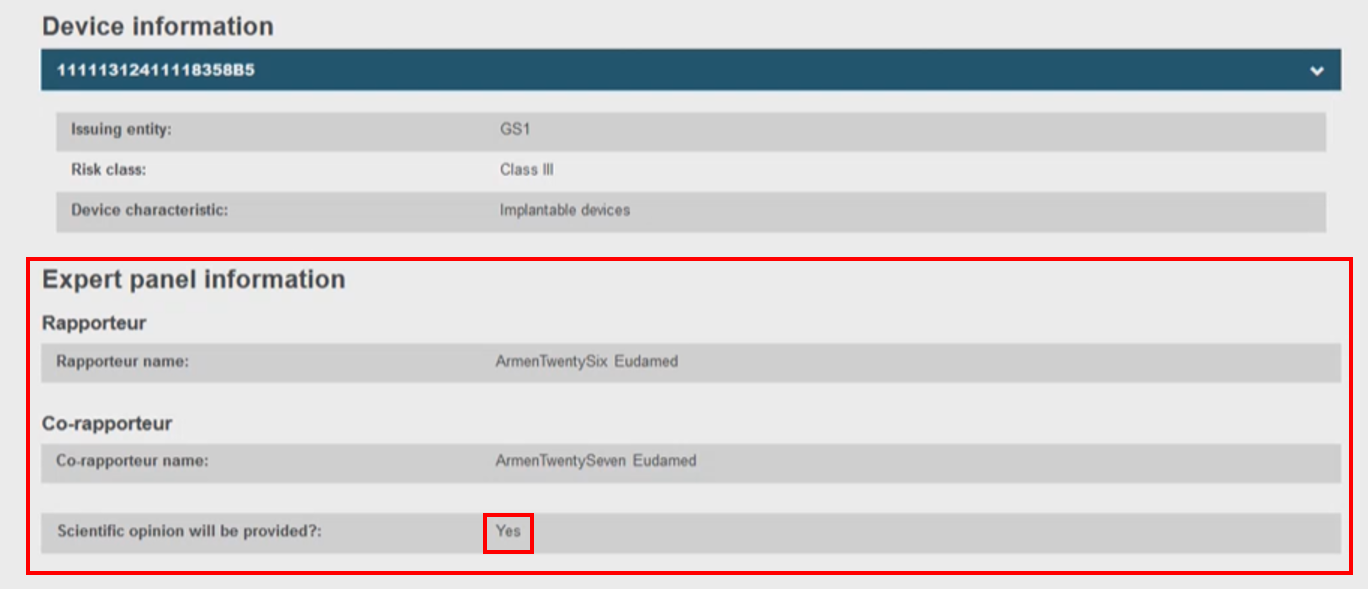
The CECP record contains the status history, manufacturer and one or more device identifiers,[17] risk class, the characteristics of the device,[18] the issuing entity if the device identifier is based on Basic UDI-DI issuer, and the NB Clinical Evaluation Assessment Report (CEAR) report.
If the CECP status is Followed or Not followed by the NB, there will be no Expert Panel Information section, otherwise for all the other CECP status there will be one.
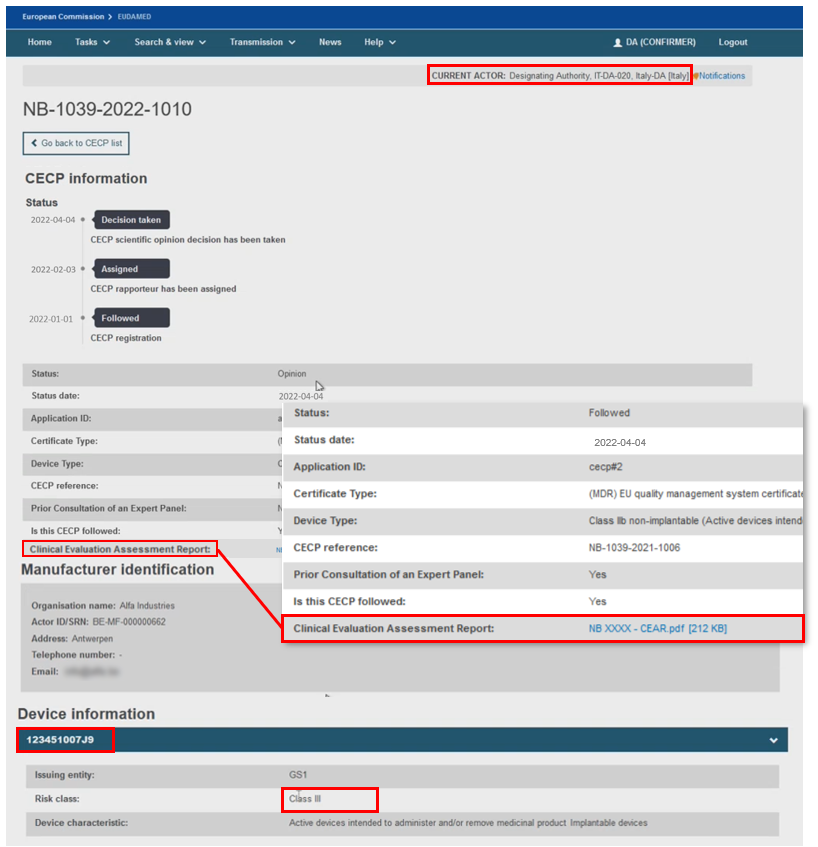 |
Information about the Expert Panel will be displayed when the CECP status is other than Followed and Not followed.
 |
[17] Device identifier can be either Basic UDI-DI or a device registered by name. Only CECP sin status Not followed may contain device identifiers registered by name.
[18] There can be Implantable for the risk class III and Not implantable, Active devices, Intended to administer and/or remove a medicinal product for risk class IIb.