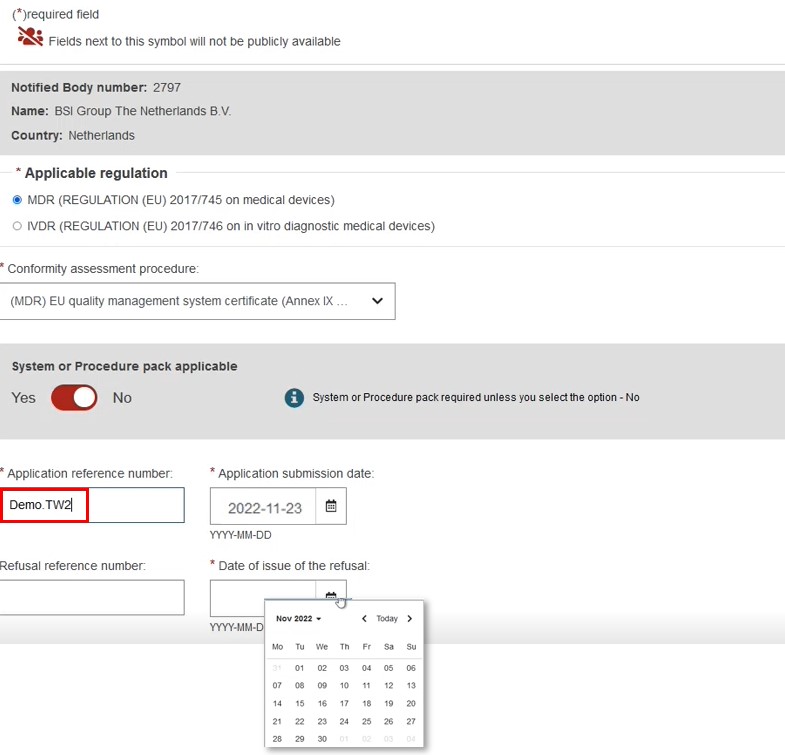
Provision of core data
Enter the core information for the refused certificate.
Select the applicable Regulation and the conformity assessment procedure.
Note
In this scenario we will choose a Quality certificate type.
Indicated Yes or No for a System Procedure Pack, then, enter the application reference number.
Provide the dates for both the application submission and the date of issue of the refusal:
Manufacturer information
Enter the Actor ID/SRN or name of the manufacturer or the system/procedure pack producer, then select the appropriate on from the displayed list:
Click Save & next.