Initial information
Indicate whether this CECP procedure is followed. For this example, we will follow the CECP – select Yes. Enter an application ID in the second field and choose the device type from the list:
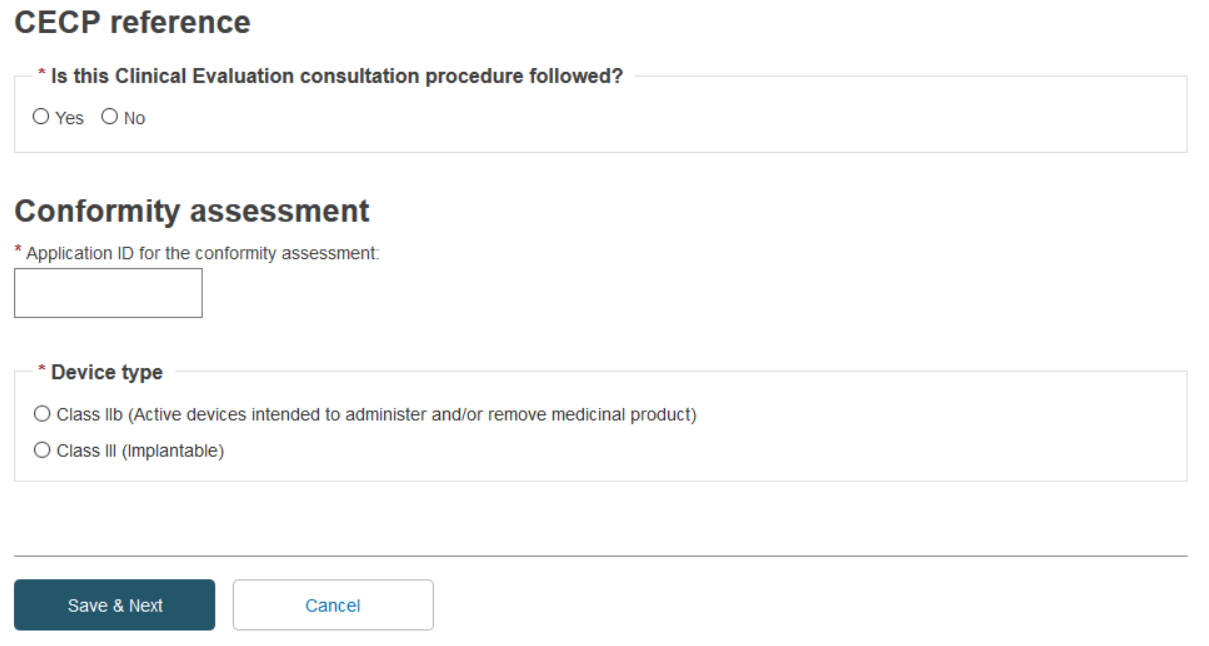
Once you have chosen your Device type, a new section Certificate type appears. Select the certificate type.
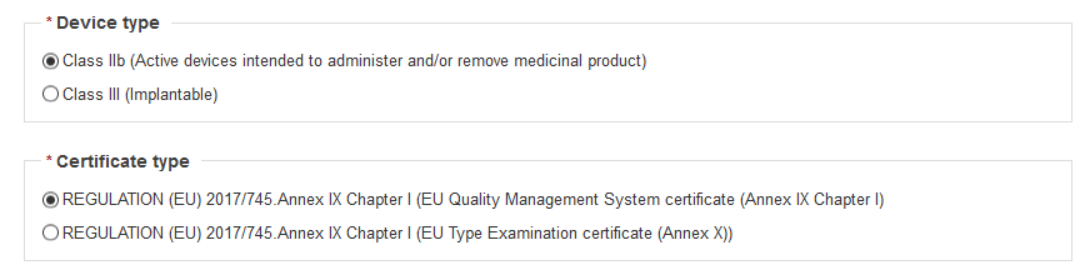
After choosing the certificate type, enter the Actor ID/SRN or name of the manufacturer.
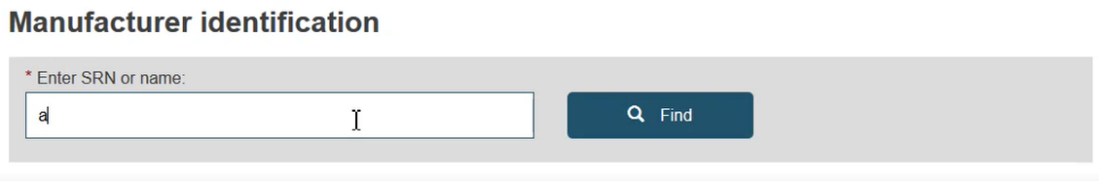
Click Find and the list of matching records is displayed.
When you select the manufacturer, its details will be displayed in the same box.
Once complete, click Save & Next.