Registering as a non-EU manufacturer
 INFOGRAPHIC: Actor registration request process
INFOGRAPHIC: Actor registration request process
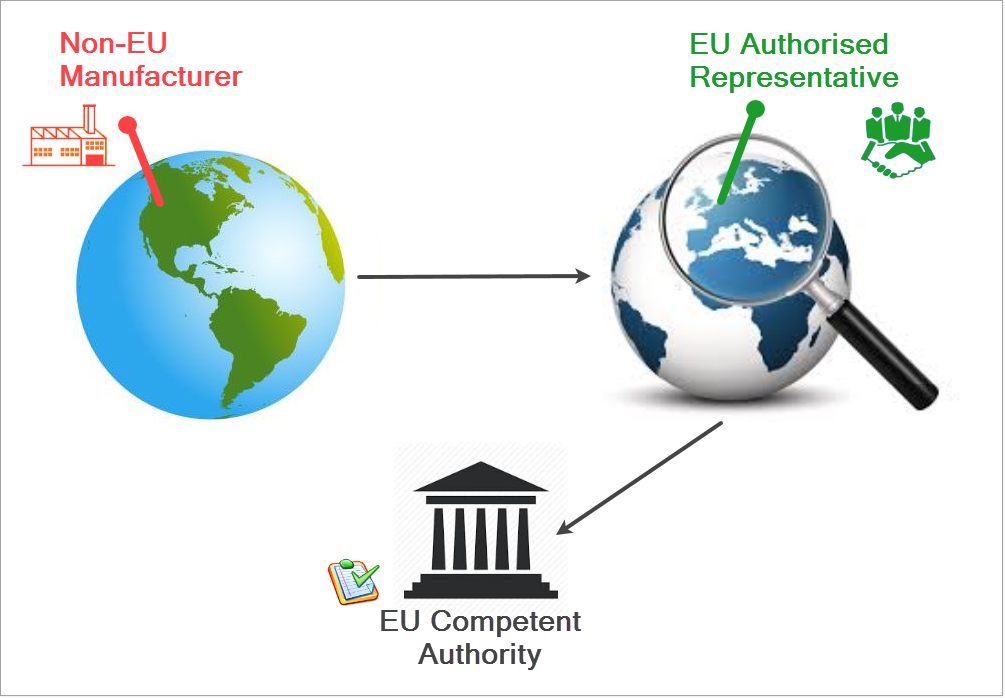
If you are a non-EU manufacturer, the procedure for registering differs from that explained in Registering as an Economic Operator.
You must identify the Authorised Representative (AR) with whom you have a mandate. If you have multiple ARs, indicate which is the main representative by its Actor ID/Single Registration Number (SRN). Your AR must verify your registration details before the request is submitted to the competent authority for validation. To do this, the AR must be registered in EUDAMED.
 |
To register a non-EU manufacturer
Log in to EUDAMED with your EU Login account.
Select the Actor registration box on the User and Actor Registration page. You are presented with an online disclaimer:
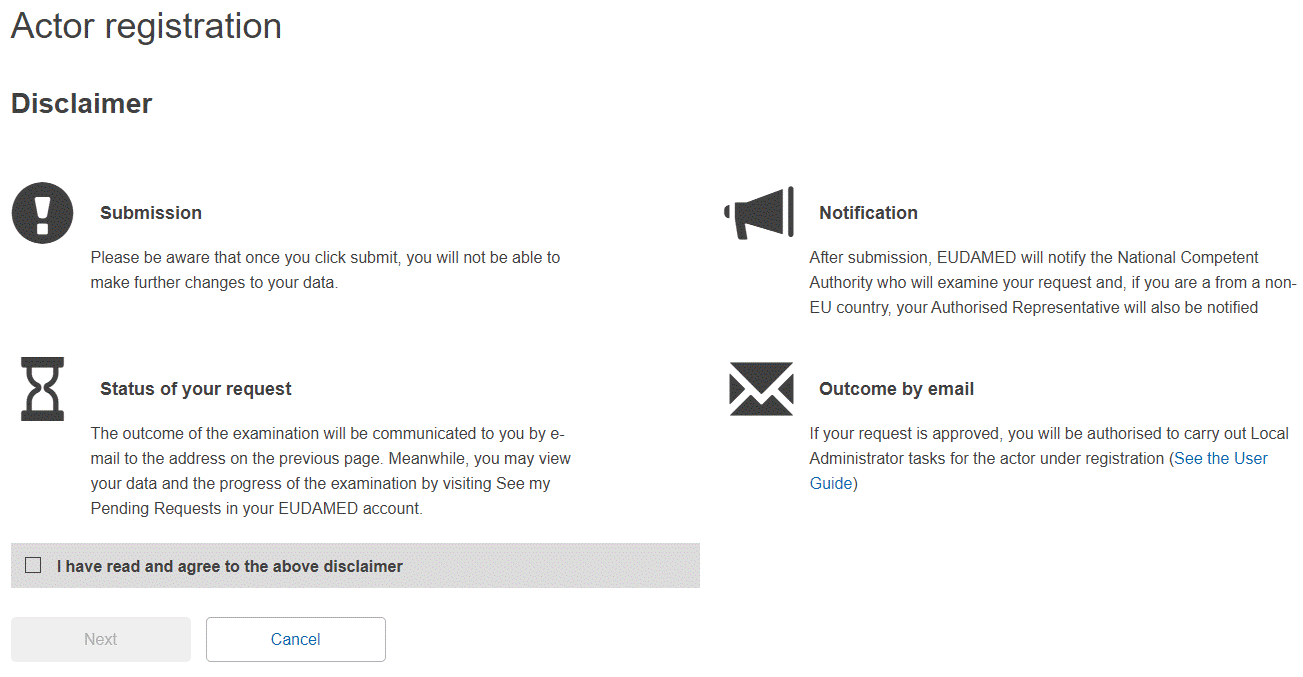
Enter the required data:
Identify your authorised representative:
Do you know the Single Registration Number (SRN)?
Yes
Select I know the SRN, click Find and select the number.
No
Deselect I know the SRN, select the country and enter the name of the Authorised Representative. Click Find and select the correct result.
Note
If you do not find your authorised representative from the search page, please contact them to confirm their Actor ID/SRN. They may not have registered yet or may have registered with a different name.
Enter the validity start and end-dates of the written mandate with the Authorised Representative.
In the Upload summary mandate document section, click Browse, select the location of the summary mandate (PDF only and not surpassing 10MB in size), and click Open. The file will appear under Upload summary mandate document. You can remove it by clicking the X next to the name of the file.
Click Save & Next.
Note
You don’t have to upload the full mandate text. It’s enough to upload a summary mandate (click here for the template).
Check and complete the information on the page. Upload the signed declaration – in PDF format only and not surpassing 10MB in size – using the Browse button.
Click Save & Next.
In the final step, the Competent Authority that will validate your registration request (i.e. the CA responsible for your Authorised Representative) is identified, with contact information.
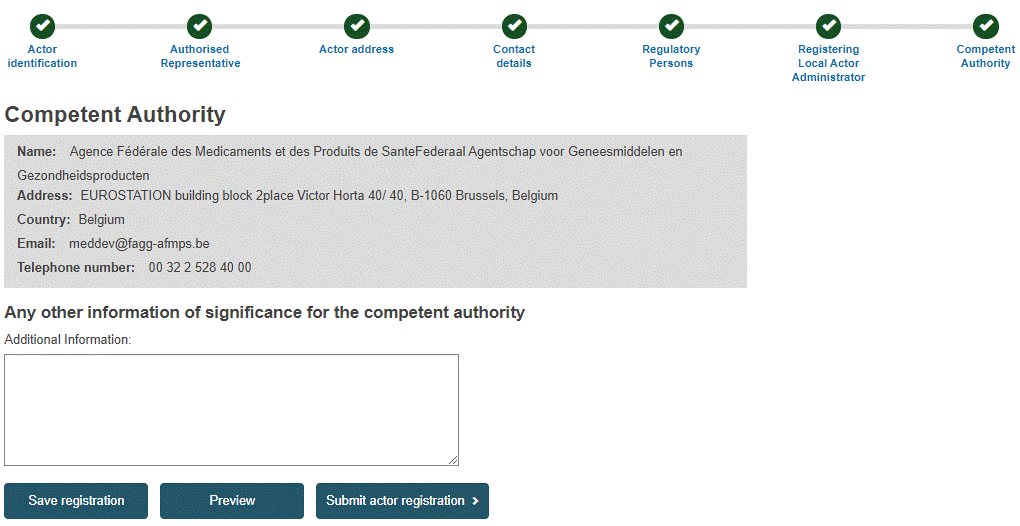
Enter any additional information you wish to pass on to the Competent Authority, and then click Preview. A summary of your completed registration form is displayed.
Review the information on the form, and then click Submit actor registration at the bottom of the page. A confirmation window will appear:
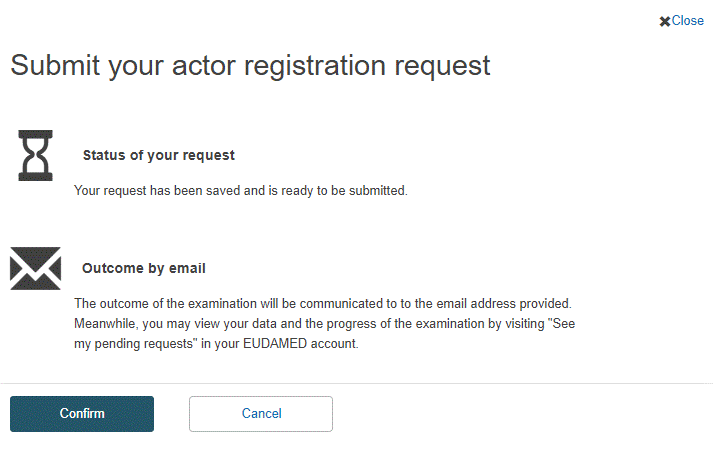
Read the information in the window and click Confirm.
Your registration request is saved and appears with a Submitted state in your Pending requests list.
Your registration request has a unique application ID and is submitted first to your Authorised Representative for verification and then to the Competent Authority for validation.
You will be notified when your Actor registration request is approved or rejected.
What next?
You can track the status of your submitted application by selecting the Home menu. The state will change to Verified once it has been verified by your Authorised Representative:
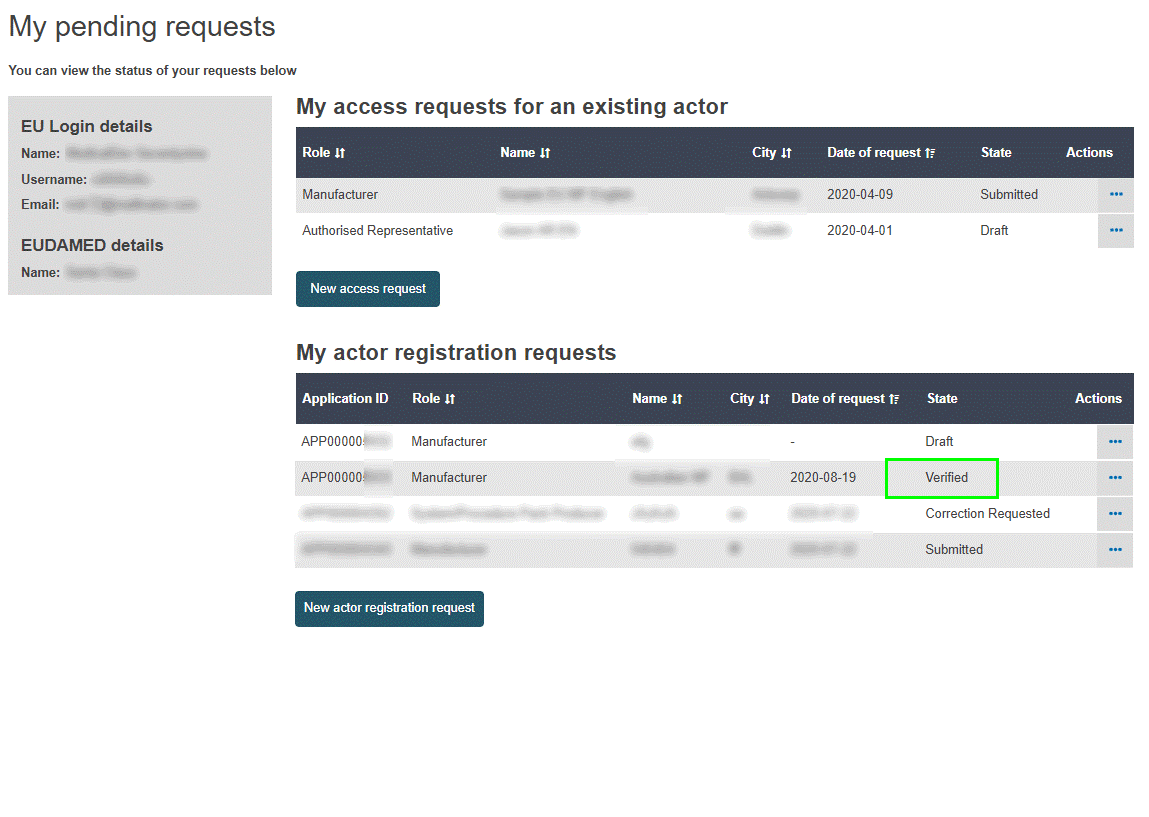 |
The Competent Authority will then assess the actor registration request. An Actor ID or Single Registration Number (SRN) is generated by EUDAMED and issued by the Competent Authority after its approval.
